
eIACUC Animal Protocol Management System
eIACUC Login [1]
The eIACUC system for submission of Animal Protocols is required for all projects utilizing a vertebrate animal species.
NOTE: A PI can choose to authorize other personnel to access, edit/amend, and start new Animal Protocols in his/her name, by utilizing the Authorizations Manager. See Managing Authorizations [2] for more information.
Training is available:
The Office of the IACUC has provided a pdf User Guide [3] to assist labs in utilizing the system. Please contact the Office of the IACUC at iacuc@uiowa.edu [4] to schedule training, and/or with any questions regarding use of the system.
Specific Help Materials for the eIACUC Animal Protocol Management System can be found at the following links:
eIACUC Navigation Assistance
Quick tips for Navigation of the eIACUC Animal Protocol Submission Program:
- The Animal Protocol is presented in several sections, which are navigated using a drop-down menu on the right of the Toolbar.
- Each section can be accessed by clicking on the section title in the menu, or using the Previous Section and Next Section buttons.
- The Animal Protocol is saved each time a new section is loaded (using the buttons or the menu).
- It can also be saved during work in progress by clicking the “Save Animal Protocol” button at the top or bottom of the page.
Answering Animal Protocol Questions
- This form includes many “smart” questions, for which the response to one question will affect the presence or absence of subsequent questions. As a result, many of the question numbers will not be sequential; this is not a mistake or problem in the form, but a way to reduce the number of irrelevant questions a user has to answer.
- Help icons and blue text links are located throughout the Animal Protocol, which link to additional information regarding the question. Clicking these icons/links will bring up a separate web browser/tab with the appropriate help material.
Dropdown Fields
- Some fields have drop-down menus which include several common responses.
- After clicking anywhere in the field or on the arrow to the right, you can then select the appropriate response by placing your cursor over it and clicking. If none of the listed responses is appropriate, choose “Other.” A text field will appear where you can enter your response.
Check Boxes
- Some questions require selection of answers using boxes which can be “checked” or “unchecked” to indicate “yes” or “no,” respectively.
- Click on either the box or the text for each appropriate answer. Multiple answers may be selected, if appropriate.
- For the Analgesia and Anesthesia sections, procedure check boxes will be populated based on the procedures provided in other sections (Nonsurgical Procedures or Surgery).
- If the procedure you are seeking to indicate in the Analgesia and/or Anesthesia section is not populated, you may need to add information on the appropriate procedure section.
Keyboard-Only Use
- If you need to move between fields without the use of a mouse (i.e. using the keyboard only), please be aware that use the “up” or “down” buttons as you navigate the page may change your selection on a dropdown menu.
- Use the “Tab” key to move between fields and the “space bar” and “enter” keys to manipulate the dropdown menus.
- Please note that hitting the “Enter” key when working in a text-entry field will activate the “Save Animal Protocol” button and the webpage will be refreshed after saving.
Text Fields
- Large text boxes can be expanded by clicking/holding the mouse button on the bars in the bottom right corner and dragging it to the appropriate size.
- In some browser/versions, this feature is not available.
- Please note that copying and pasting text into the text box will not retain any formatting (bold/underline/italicized font, page breaks, images, etc.)
Repeated Question Sets
- Many of the sections include repeatable sets of questions which pertain to a specific topic (a person, procedure, drug, etc.).
- These question sets allow answers to be provided for each individual item, and additional entries can be added until all are listed.
- Each question set is indicated by a black box surrounding the repeated area.
- For some sections, when more than one repeated set has been answered, the entries will “collapse” to make the page easy to read and navigate.
- To expand a collapsed entry, click the header or the arrowhead to the left of the desired entry.
- To add a new entry, click the “Add” button below the last question set; to delete an entry, click the “Delete” button at the bottom of the question set for that entry.
Personnel Sections
- In the three personnel sections (Principal Investigator, Co-Principal Investigator, and Technical/Student Personnel), the first question will search the University of Iowa Directory for the person being added.
- Enter the HawkID or at least three letters of the last name of the person to be added. A spinning icon will appear to indicate that the program is searching.
- Select the appropriate person from the list of names that appears.
- If too many names are found, the search can be refined by adding additional characters or adding a comma after the last name, followed by the first initial/name (e.g. Doe, John)
- Once a name from the list is selected, many of the fields will be pre-populated with that person’s information from the University of Iowa Directory.
- Confirm that all details are correct and revise/add information as appropriate.
- The Animal Use Education Course and Occupational Health Questionnaire information will be automatically populated from electronic records. If this information is incomplete for one or more person, the Animal Protocol can be submitted for review; however, final IACUC approval cannot be granted until the appropriate training and information is collected for all personnel.
Protocol Summary Page Menu Options:
- Edit – Make changes and/or submit an in-progress draft Animal Protocol or draft Amendment
- Compare – Compare an Amendment submission with a previous Animal Protocol version
- Amend – Start an Amendment to an approved Animal Protocol (Note: only one Amendment in Draft or In Review status is permitted for each Animal Protocol)
- Renew – Create a copy of an existing Animal Protocol for the purposes of continuing the same project
- Copy – Create a copy of an existing Animal Protocol to edit and submit as a new Animal Protocol (including Duplicate Submissions for funding purposes)
- View – Read-only access for the purpose of viewing and/or printing the Animal Protocol
- Withdraw – Withdraw an Animal Protocol or Amendment which is under IACUC review. This will revert the status from In Review to Draft, and allow the submission to be deleted. This option should only be used in circumstances where a PI no longer wishes for the Animal Protocol or Amendment to be submitted to the IACUC.
- Delete – Permanently delete an Animal Protocol or Amendment. This can only be done for a form in Draft status. This action cannot be reversed.
For full detailed directions of the eIACUC Program, please see the eIACUC User Guide [3].
eIACUC Animal Protocol User Guide
To view, download, and/or print the eIACUC User Guide, click here: ![]() eIACUC User Guide.pdf [5]
eIACUC User Guide.pdf [5]
Managing Authorizations
A PI can choose to authorize other personnel to access, edit/amend, and start new Animal Protocols in his/her name, by utilizing the Authorizations Manager.
The PI can also choose to grant one or more individual(s) permissions to make authorizations to other personnel. This permission level allows the authorized individual to perform many tasks on the PI’s behalf; however, the PI remains ultimately responsible for all activity performed under the Animal Protocol.
Authorizations Summary Page:
- Select “Authorizations” from the eIACUC Toolbar to access the Authorizations Summary page.
- The Authorizations Summary page provides a list of all Animal Protocols to which you have any level of access.
- Click the arrowhead to the left of an Animal Protocol number to view all personnel with access to that AP, including their access level.
To adjust the access level of any person listed on your Animal Protocol (or grant access to an individual not listed as personnel on your Animal Protocol), click the “Go to Authorizations Manager” button at the top of the Authorizations Summary page.
Authorizations Manager:
There are four access levels that can be selected:
- No Access: This person is listed on the Animal Protocol, but cannot access it via eIACUC.
- View Only: This person can view and print the Animal Protocol, but cannot make changes to drafts or create Amendments.
- View and Edit: This person can view/print, edit drafts, create Amendments, and submit the Animal Protocol/Amendment for IACUC review.
- Authorize Others: This person can manage authorizations on this Animal Protocol, in addition to all View and Edit actions.
All personnel listed on one (or more) of your existing Animal Protocol(s) will be initially granted “View Only” access to the Animal Protocol(s) on which they are listed.
-
To change Authorizations for a listed person:
- Click the arrowhead to the left of the person’s name to expand his/her Authorization details
- Select the drop down at the top of the table to set a default access (applicable to all Animal Protocols listed).
- Individual authorization levels can be managed by adjusting the drop-down menu to the right of each Animal Protocol in the table.
- If the desired Animal Protocol to which you want to grant access does not appear (for instance, if a new AP has been added after access levels have been set for a person), click the “Update Protocol List” button at the top of the table to refresh the list of APs for which you can manage authorizations.
-
To grant access to an individual not listed on any of your Animal Protocols:
- Use the “Authorize User” personnel selection box at the top of the page to search for the desired person by last name or hawkid.
- This selection box functions in a similar manner to those in the Personnel Sections.
- Once the appropriate person has been selected, click “Add Authorizations.”
- An entry table for that person will appear in the list below and authorizations can be managed as above.
- Use the “Authorize User” personnel selection box at the top of the page to search for the desired person by last name or hawkid.
-
To grant a person permission to start new Animal Protocols in your name (as the PI):
- Select the “check box” above the AP list in that person’s authorizations table (allow user to act as your proxy)
- This gives permission to create a new AP from a blank form and to renew or copy an existing AP.
Log Out of eIACUC
How do I log out of eIACUC?
Because eIACUC provides access to multiple systems using a single login, you will need to end your session by closing your browser. This will ensure that anyone that uses your computer will not automatically be logged in as you when they use your browser to go to research administration websites.
Note: Logging out of other research administration sites may disrupt your single sign-on experience.
The eIACUC system allows you to sign-on to one site and navigate between research administration sites without logging on again. Logging out of other research administration sites will end your session on on that site but not on other research sites such as eIACUC. If you have a window open to UIRIS, HawkIRB, or other sites you reached via the portal you will continue to be able to use them, but clicking on a link to the site you logged off of will require you to log in. Always close your browser when ending a session with your research administration sites.
Animal Protocol Agent Administration Table
The Non-surgical section of the eIACUC Animal Protocol form includes instruction to complete a table for any agents to be administered to the animal. The table includes columns for each piece of required information: Animal Protocol title, PI name, species, agent name, dose, volume, route, any potential adverse events known from the agent, and whether or not the agent is pharmaceutical grade. Diluents and vehicle controls should be included. Analgesics, anesthetics, paralytics, and euthanasia agents should not be included.
A blank form can be found here:
![]() Agent Administration Table Template.xlsx [6]
Agent Administration Table Template.xlsx [6]
Example (Filled) Agent Administration Table can be viewed here:
Examples of Experimental Design Summary
Example 1:
Each group of animals will undergo surgery to place a minipump subcutaneously. Some of these animals will also have an intracerebral cannula connected to the minipump for drug delivery, while the others will have the drug delivered subcutaneously. After two to eight weeks of drug administration, groups may begin behavioral studies (procedure A, B, C, or D), which may occur as frequently as daily for up to 4 weeks, or twice weekly for up to 6 months. Some animals may undergo a second surgical procedure to replace the minipump after 4 weeks of delivery, to provide a total of 8 weeks of continuous drug administration. Some animals will be euthanized at intervals after drug delivery without further testing, while others will be euthanized following behavioral testing of up to 6 months. The maximum time from the start of studies to euthanasia will be 8 months.
Example 2:
Each group of animals will be administered drugs by various routes over the course of up to 3 months (see drug table). Following at least one week of drug administration, blood pressure samples will be collected using noninvasive methods. Once the drug administration period is completed (up to 3 months after starting), some animals will undergo a non-survival surgery procedure for surgical blood pressure sampling procedures at various times after the last drug dose. Other groups will be euthanized and tissues collected for further analysis. Time from start of drug administration to euthanasia will be no more than 1 year.
Example 3:
Project 1) Groups of animals will be inoculated with 5 different doses of Example Virus or vehicle, with or without the addition of Example Drug A, B, or C at the time of injection. After appearance of clinical disease, some animals will receive treatment with experimental agents D, E, F, or G over the course of up to 12 weeks. Other animals will receive vehicle alone or no treatment. At time points up to 24 weeks after initial inoculation, animals will be euthanized and tissues collected.
Project 2) Animals will undergo one of 4 surgical procedures (Procedure A, B, C, or D) followed by treatment with Example Drug A, B, or C at the time of surgery. Some animals will receive treatments with agents D, E, or F as detailed above, and euthanized at up to 2 years following initial surgery.
Project 3) Animals will be given medicated feed to induce gene expression for up to 10 weeks before inoculation with Example Virus with or without Example Drug A as described in Project 1. Over the following 6 weeks, clinical signs will be evaluated and animals euthanized at various time points for tissue analysis. Some animals will undergo non-survival procedure A to analyze immune reactions, after which they will be euthanized without recovery from anesthesia. Animals will be maintained for up to 15 months post-infection before euthanasia.
Example 4:
Animals will be purchased and euthanized for the postmortem collection of tissues. No other procedures will be performed.
Example 5:
Groups of animals will receive drug treatments by injection or in special feed for up to 8 weeks, after which they will be euthanized and tissues collected for further analysis.
Contact for eIACUC Help
E-mail: iacuc@uiowa.edu [4]
Phone: (319) 335-7985
Institutional Animal Care and Use Committee
L350 Pappajohn Biomedical Discovery Building (PBDB)
Iowa City, Iowa 52242
Help Information for Specific Animal Protocol Questions
A. Changes in Scope of Grant
- Change in the specific aims approved at the time of award
- Substitution of one animal model for another
- Change from the approved use of live vertebrate animals
- Shift of the research emphasis from one disease area to another
- Application of a new technology, e.g. changing assays from those approved to a different type of assay
B. Funding
C. Is this person performing surgery?
D. Instructional Course
E. Literature Search
F. Overall/Program/Training Protocol Definitions
Overall Project: When a large project grant gets funded, only a portion of it may involve animal work. The money may come into a University Center or an individual PI (i.e. main awardee) who has various PIs responsible for certain projects under the main grant. The main grant (encompassing all projects) is typically tied to what is called an “Overall” Animal Protocol approval. The Overall is a sort of landing-place for the grant, and does not outline any animal work. The specific projects involving animal work are tied to separate Animal Protocol approvals under the corresponding PIs.
Center/Program project: A grant for which there is a common theme (e.g. heart disease) but for which there are multiple projects that may be done by any number of Co-PIs under the main grant. The grant typically has a Primary awardee and comes into the University as a “Center/Program grant”. The grant will have a “P” such as P01, P20, etc in the grant ID number. The main grant (encompassing all projects) is typically tied to what is called an “Overall” Animal Protocol approval. The Overall is a sort of landing-place for the grant, and does not outline any animal work. The specific projects involving animal work are tied to separate Animal Protocol approvals under their corresponding PIs. The PIs involved in this type of grant, should refer to the Center/Program grant and the PI named as the primary PI (so that the funds can be appropriately followed back to the main grant).
Training Grant: A grant that geared to training students, post-docs, faculty, etc for which there is a common theme (e.g. heart disease). Only a portion of the grant may involve animal work. The money may come into a University Center and subsequently be distributed to the various PIs responsible for certain projects. The grant will have a “T” such as T32 in the grant ID number. The main grant (encompassing all projects) is typically tied to what is called an “Overall” Animal Protocol approval. The Overall is a sort of landing-place for the grant, and does not outline any animal work. The specific projects involving animal work are tied to separate Animal Protocol approvals under their corresponding PIs. The PIs involved in this type of grant, should refer to their protocol as a Training grant.
G. Animal Housing Location
H. Adverse Phenotype
- Frequent deaths around weaning age due to small size at normal weaning age (21 days old)
- Delay weaning until 28 days old (or longer if needed)
- Provide gruel in breeding cage for one week prior to weaning
- Paresis or paralysis
- Separate affected animals from cage-mates that are unaffected (to reduce competition for food and water resources)
- Provide gruel daily on cage floor for easier access to food
- Spontaneous tumor development
- Gruel may be provided daily if tumors lead to cachexia (muscle wasting/decreased body condition)
- Managed by increased monitoring and adhering to humane endpoint limits
- Ulcerative skin lesions (ulcerative dermatitis)
- Provide daily supplement of Vitamin E and omega fatty acids (DermaForm liquid) provided on gruel in a dish on the cage floor – Recommended
I. Drugs and Substances
J. Will any special husbandry or housing conditions be required for this project?
K. Surgical Plane of Anesthesia
Anesthesia has been described as a series of four Stages.
- Stage 1: the period between administration of an anesthetic and loss of consciousness.
- Stage 2: the period after loss of consciousness, which may include actions such as uncontrolled movement, delirium, vocalization.
- Stage 3: the level at which surgery can be performed. Stage 3 anesthesia is divided into four planes.
- Plane 1: "light" anesthesia - the animal still has blink and swallowing reflexes, and regular respiration.
- Plane 2: "surgical" anesthesia - the animal has lost blink reflexes, pupils become fixed and respiration is regular.
- Plane 3: "deep" anesthesia - the animal starts losing the ability to use the respiratory muscles and breathing becomes shallow; may require assisted ventilation.
- Plane 4: the animal loses all respiratory effort, and breathing may stop entirely.
- Stage 4: Irreversible Anesthesia—respiratory arrest, followed by circulatory collapse.
L. Death as an Endpoint
M. Hazards
Guidance for identification of hazardous substances used in projects
Some hazardous chemicals or drugs fall into multiple classifications/categories. List the substance in the category that, based on your review, fits best. You may specify multiple categories if appropriate. Include ALL substances that fit the categories below, regardless of containment status (inclusion in this list does not automatically require additional containment or special handling).
Human or non-human primate blood, blood products, tissues, tumors, and/or cells:
Include any cell, tissue, or blood product of human or non-human primate origin, including cells/tissues growing in culture.
Other animal blood, blood products, tissues, tumors, and/or cells:
Include any animal cell, tissue, or blood product which does not fall under the human or non-human primate category above.
Engineered Nanomaterials/Nanoparticles
Include any engineered nanomaterial/nanoparticle (size range 1-100 nanometers). Include the name of the material, the particle size/dimensions, and the form of the material administered to animals such as aerosol, liquid suspension, etc. Also include whether or not the materials is believed to be biodegradable. Limited examples include carbon nanotubes, titanium dioxide, polystyrene.
Toxic chemicals, including carcinogens
| OSHA Hazard Classification | OSHA Hazard Category | OSHA Pictogram | Signal Word on Label or SDS | Hazard Statement on Label or SDS |
|---|---|---|---|---|
| Carcinogenicity | 1A, 1B |  |
Danger | May cause cancer |
| Carcinogenicity | 2 |  |
Warning | Suspected of causing cancer |
| Acute Toxicity, Oral | 1,2 |  |
Danger | Fatal if swallowed |
| Acute Toxicity, Oral | 3 |  |
Danger | Toxic if swallowed |
| Acute Toxicity, Dermal | 1,2 |  |
Danger | Fatal in contact with skin |
| Acute Toxicity, Dermal | 3 |  |
Danger | Toxic in contact with skin |
| Acute Toxicity, Inhalation | 1,2 |  |
Danger | Fatal if inhaled |
| Acute Toxicity, Inhalation | 3 |  |
Danger | Toxic if inhaled |
| Skin Corrosion/Irritation | 1A, 1B, 1C | 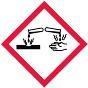 |
Danger | Causes severe skin burns and eye damage |
| Eye Damage/Irritation | 1 | 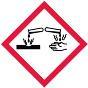 |
Danger | Causes serious eye damage |
| Sensitization, Respiratory | 1A, 1B |  |
Danger | May cause allergy or asthma symptoms or breathing difficulties if inhaled |
| Sensitization, Skin | 1A, 1B |  |
Warning | May cause an allergic skin reaction |
| Germ Cell Mutagenicity | 1A, 1B |  |
Danger | May cause genetic defects |
| Specific Target Organ Toxicity, single exposure | 1 |  |
Danger | Causes damage to organs (specific organs may be listed) |
| Specific Target Organ Toxicity, single exposure | 2 |  |
Warning | May cause damage to organs (specific organs may be listed) |
| Specific Target Organ Toxicity, repeated exposure | 1 |  |
Danger | Causes damage to organs though prolonged or repeated exposure |
| Aspiration hazard | 1 |  |
Danger | May be fatal if swallowed and enters airways |
Limited examples include heavy metals, PCBs, neurotoxins, pesticides, benzene, DMBA, cyanide compounds, urethane, isocyanates
Examples may be found in the following references (carcinogenicity):
California Proposition 65 List of Chemicals (known to cause cancer or reproductive toxicity) [11]
National Toxicology Program (NTP), “Report on Carcinogens” (latest edition) [12]
International Agency for Research on Cancer (IARC), “Monographs on the Evaluation of Carcinogenic Risks to Humans” (latest editions) [13]
OSHA 29 CFR part 1910, Subpart Z, Toxic and Hazardous Substances
Hazardous drugs including chemotherapy/antineoplastic and investigational drugs
Using the NIOSH definition, drugs considered hazardous include those that exhibit one or more of the following six characteristics in humans or animals:
- Carcinogenicity
- Teratogenicity or other developmental toxicity
- Reproductive toxicity
- Organ toxicity at low doses
- Genotoxicity
- Structure and toxicity profiles of new drugs that mimic existing drugs determined hazardous by the above criteria
Examples may be found in the following references:
NIOSH publication NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2014 or most current [14]
OSHA Technical Manual “Controlling Occupational Exposure to Hazardous Drugs”
Appendix VI:2-1. Some Common Drugs Considered Hazardous [15]
| OSHA Hazard Classification | OSHA Hazard Category | OSHA Pictogram | Signal Word on Label or SDS | Hazard Statement on Label or SDS |
|---|---|---|---|---|
| Carcinogenicity | 1A, 1B |  |
Danger | May cause cancer |
| Carcinogenicity | 2 |  |
Warning | Suspected of causing cancer |
Limited examples include doxorubicin, bleomycin sulfate, streptozotocin, cisplatin, tamoxifen, paclitaxel
Note that certain pharmaceuticals used in their common pharmaceutical form may be exempted at the discretion of the IACUC although they may have hazards such as carcinogenicity or reproductive hazards associated with their use.
Additional examples may be found in the following references:
National Toxicology Program (NTP), “Report on Carcinogens” (latest edition) [16]
International Agency for Research on Cancer (IARC), “Monographs on the Evaluation of Carcinogenic Risks to Humans” (latest editions) [17]
OSHA 29 CFR part 1910, Subpart Z, Toxic and Hazardous Substances
Reproductive hazards
Include mutagens, teratogens, developmental reproductive toxicity
| OSHA Hazard Classification | OSHA Hazard Category | OSHA Pictogram | Signal Word on Label or SDS | Hazard Statement on Label or SDS |
|---|---|---|---|---|
| Germ Cell Mutagenicity | 1A, 1B |  |
Danger | May cause genetic defects |
| Toxic to Reproduction | 1A, 1B |  |
Danger | May damage fertility or the unborn child |
| Toxic to Reproduction | 2 |  |
Warning | Suspected of damaging fertility or the unborn child |
Limited examples include BrdU, oxytetracycline hydrochloride, RU-486 (mifepristone), 5-FU (fluorouracil), ribavirin, bleomycin sulfate, tamoxifen citrate
Examples may be found in the following reference:
California Proposition 65 List of Chemicals (known to cause cancer or reproductive toxicity) [11]
Note that certain pharmaceuticals used in their common pharmaceutical form may be exempted at the discretion of the IACUC although they may have hazards such as carcinogenicity or reproductive hazards associated with their use.
Other potential or known hazardous substance (chemical)
Air-reactive, water-reactive, self-reactive other highly reactive chemicals with OSHA Signal Word “Danger”
Explosive chemicals with OSHA Signal Word “Danger”
Organic Peroxides with OSHA Signal Word “Danger”
Flammable Gases, Liquids, Aerosols, Solids with OSHA Signal Word “Danger”
Oxidizing Gases, Liquids, Solids with OSHA Signal Word “Danger”
N. Amendment Summary
Provide a brief summary of the purpose of the Animal Protocol amendment to be submitted. For example: "This amendment is needed to add 2 new procedures." or "This amendment is to increase animal numbers to achieve statistical significance following a pilot study, and to add additional personnel."
O. Procedures for Analgesics
The procedures listed under Question #16 are populated from the "Non-surgical Procedures" and "Surgery" sections, respectively. Some procedures which are populated may not be those perceived to cause pain or distress. If a surgical or non-surgical procedure is not listed in Question #14, confirm that it has been entered under the appropriate section. If analgesia is to be administered for circumstances other than those populated in Question #14, describe it under Question #16.
P. Procedures for Anesthesia
The procedures listed under Question #12 are populated from the "Non-surgical Procedures" and "Surgery" sections, respectively. Some procedures which are populated may not be those perceived to cause pain or distress. If a surgical or non-surgical procedure is not listed in Question #10, confirm that it has been entered under the appropriate section. If anesthesia is to be administered for circumstances other than those populated in Question #10, describe it under Question #12.
Q. Age Definitions for Euthanasia
For the purposes of euthanasia, rodents are grouped into 3 different age groups: fetus, neonate, and adult. The requirements for performance and/or confirmation of euthanasia procedures will depend upon the age of the animal. The age at which an animal moves from one group definition to another depends on the species.
Mice, rats, gerbils, and hamsters:
- Feti greater than 15 days' gestation require confirmation of euthanasia (See IACUC Guidelines on Confirmation of Euthanasia).
- Neonates up to 10 days post birth are treated as neonates for euthanasia purposes (See IACUC Guidelines on Euthanasia).
- Animals greater than 10 days of age are treated as adults for euthanasia purposes.
Guinea pigs:
- Feti greater than 35 days' gestation require confirmation of euthanasia (See IACUC Guidelines on Confirmation of Euthanasia).
- Animals are treated as adults for euthanasia purposes starting at birth (no "Neonate" classification for euthanasia purposes).
R. Housing at Animal Biosafety Level 1 (ABSL-1)
Standard OAR housing (under either barrier or non-barrier conditions) is considered ABSL-1. These animals are treated with standard universal precautions; exposure to the animals and any agents which may be administered to them, is not known to cause disease in immunocompetent adult humans, and there is minimal potential hazard posed to personnel and the environment. This is in contrast to animals housed at ABSL-2, or -3, which are infected with agents capable of causing human or animal disease or are of unknown risk; these animals are handled with special measures, including procedures, equipment, and decontamination procedures, in order to control the risk of exposure. In the case of recombinant or synthetic nucleic acids, if you are unsure whether your rodents should be handled with increased containment measures, please contact the IBC at ehs-rdna@uiowa.edu [18] or 353-5679.
S. Start New Animal Protocol
Clicking the "New Animal Protocol" button will create a new blank form to be completed for the purposes of describing animal research/teaching activities. Please see When is an Animal Protocol Required? [19] and/or contact the IACUC office at iacuc@uiowa.edu [4] if you need more information on the conditions which require an IACUC-approved Animal Protocol.
T. Find Existing Animal Protocol
You may wish to locate an existing Animal Protocol for the purposes of viewing the Animal Protocol, creating an Amendment, renewing or copying the Animal Protocol, or editing a draft version. Animal Protocols may be located by entering one or more search terms in the appropriate box: Animal Protocol number, the PI name or the name of any personnel listed on the Animal Protocol, the Project Title (or portion thereof), and/or filter the Animal Protocols which you are authorized to view using any of the checkboxes or filter options available. Once you have entered the appropriate information, click the "Search" button to produce the Animal Protocol(s) which meet your criteria.
You may then select the appropriate Animal Protocol by clicking the blue Animal Protocol number on the left side of the table. Clicking this link will produce the Protocol Summary page, from which you may select subsequent actions (View, Edit, etc.)
U. Animal Protocol Actions
- View: This action allows a user to view all sections of the Animal Protocol. A printable pdf may also be produced by accessing the Animal Protocol using this action.
- Edit: This action allows an authorized user to make and save edits to any section of the Animal Protocol or Amendment currently in Draft or Revisions status.
- Compare: This action allows a user to compare the most recent version of the Animal Protocol to a previous version (Draft, Approved, etc.). This action may be useful for determining what changes have been made during an Amendment proposal.
- Amend: This action allows an authorized user to begin a Draft Amendment to an Approved Animal Protocol. This action is only available on an Approved Animal Protocol for which an amendment is not currently in Draft or under IACUC review. Any number of modifications can be made in a given amendment request; however, only one amendment request can be initiated/in review at a time.
- Renew: This action allows an authorized user to start a Renewal Animal Protocol from the currently approved version of an Animal Protocol. All pertinent information (including Project Title, Animal Protocol Number and funding information) will be transferred to the new draft. All appropriate edits may then be made in the newly created Renewal Animal Protocol prior to submission for IACUC review.
- Copy: This action allows an authorized user to start a new Animal Protocol by copying the majority of an existing Animal Protocol. All sections will be copied, except for pertinent information in the Project Information section (including Project Title, Animal Protocol Number and funding information). All appropriate edits may then be made in the newly created Animal Protocol prior to submission for IACUC review.
- Withdraw: This action allows an authorized user to withdraw a submitted Animal Protocol or Amendment currently under IACUC review. Withdrawing a submitted document will remove it from the review process entirely.
- Delete: This action allows an authorized user to delete a Draft version of an Animal Protocol or Amendment. Once an Animal Protocol or Amendment is under IACUC review, it cannot be deleted without first being withdrawn.
V. Personnel Experience
The following are examples of how to adequately address experience for personnel included in the Animal Protocol.
(Non-surgical experience question) - For each species used, describe training and experience with Non -surgical techniques and procedures, or explain how he/she will be trained and by whom.
- John has 5 years of experience conducting experiments with rats. He has experience with some of the non-surgical (or surgical procedures) in this protocol and will be trained by experienced lab members before conducting any of the other approved procedures.
- John has performed euthanasia of mice by microwave irradiation for 5 years and will train others in this technique.
- John has not worked with dogs and does not intend to do so at this time.
(Surgical experience question) - For each species used, describe training and experience with the proposed surgical procedure(s) or explain how he/she will be trained and by whom.
- John has been performing surgery in mice and rats for greater than 5 years and has experience with some of the surgical procedures in this protocol and will be trained by experienced lab members before conducting any of the other approved procedures.
- John is being trained to assist the surgeon with equipment during dog surgery but will not perform any procedures on dogs.
W. Training Animals
Training animals are used in cases where individuals need to develop skills and become competent in procedures, in addition to those used to refine a procedure. Includes an animal carcass or an animla used in a terminal/non-survival procedure.
- An IACUC approved Animal Protocol is required to use training animals
- Training animals reach their humane endpoint when the training objective is complete; they are not used to collect subsequent data
- Different humane endpoints may be needed for training than for data collection