Policies and Guidelines
The IACUC and the Office of Animal Resources have provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa.
- Policy - An exception to an IACUC Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
- Guideline - An exception to an IACUC Guideline must be described and justified in the Animal Protocol and approved during the normal approval process.
- Informational Sheets provide information about frequently asked questions and represent guidance for best practices. Deviation from the recommendation(s) does not require specific justification but clarification may be requested during the review process.
Analgesia (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose
The purpose of these guidelines is to describe appropriate analgesia regimens for the management of pain in animals used in teaching, research and testing at the University of Iowa. These guidelines include minimum analgesia recommendations. Animals should be monitored for an appropriate time period to determine if analgesia provisions are adequate. Any animal showing evidence of pain should be provided analgesia. If analgesia cannot be provided due to scientific reasons, the rationale should be described and approved in the Animal Protocol.
Recognition of Pain
Adequate alleviation of pain in laboratory animals requires the training and knowledge to recognize signs of pain which may differ between species. An information sheet titled “Pain Recognition in Laboratory Animals” is provided for reference, and veterinary consultation is available for personnel training and advice on pain recognition in unique models.
Pre-Emptive Use of Analgesic Agents
Pre-emptive analgesia should be provided whenever possible. Analgesia provisions are most effective at reducing the intensity of painful stimulation when given prior to the painful event.
- Advantages of pre-emptive use of analgesics:
- Reduces the intensity of painful stimulation
- Improves the animal's comfort level after surgery
- Decreases the amount of anesthesia required to maintain a surgical plane
- Results in a smoother recovery
Multi-Modal Use of Analgesic Agents
- Multi-modal analgesia (giving multiple drugs with different mechanisms of action) provides the best analgesia possible
- e.g., local analgesia + opioid + NSAID (when appropriate)
- Classes, dosages, routes and frequency of administration are listed in the “Common Analgesic Agents” section of these guidelines
Minimum Analgesia Requirements for Mammals
- Subcutaneous wounding, implantations or procedures without incising through muscle wall:
- NSAIDS
- Analgesia should be provided for a minimum of 24 hours post-op
- Incisions into retro-peritoneal or abdominal cavity or the muscle wall:
- NSAIDS and/or Opioids
- Analgesia should be provided for a minimum of 48 hours post-op
- Incisions into thoracic cavity through the muscle wall:
- Opioids
- Analgesia should be provided for a minimum of 48 hours post-op
- Craniotomy
- NSAIDS and/or Opioids
- Analgesia should be provided for a minimum of 48 hours post-op
Local Anesthesia Along Intended Incisions (Line Blocks)
Table: Formulary for rats and mice
|
Local Anesthetic |
Dose |
Application |
Notes |
|---|---|---|---|
|
Lidocaine |
Dilute to 0.5%, do not exceed 7mg/kg total dose; Incisional line block |
Inject locally before surgical incision |
Faster onset than bupivacaine (2-3 minutes after injection) but short (<1hour) duration of action |
|
Bupivacaine |
Dilute to .25%, do not exceed 8mg/kg total dose; incisional line block |
Inject locally before surgical incision |
Slower onset than lidocaine (20+ minutes) but longer (4-8 hour) duration of action |
|
0.5% Lidocaine/0.25% Bupivacaine mixture |
See formulation below; do not exceed maximum doses |
Inject locally before surgical incision |
Best option – rapid action of lidocaine with prolonged action of bupivacaine |
Dilutions:
- Lidocaine
- Dilute the 2% (20 mg/ml) Lidocaine 1:4 to get final concentration of 0.5% (5 mg/ml)
- Example: 0.5 mL of 2% lidocaine + 1.5 mL sterile saline
- Label with drug, concentration, and expiration date
- See “IACUC Guidelines: Use of Drugs and Chemicals in Laboratory Animals” for further details (proper handling, expiration guidance, etc.)
- Bupivacaine
- Dilute the 0.5% (5 mg/ml) Bupivacaine 1:2 to get final concentration of 0.25%
- Example: 0.5 mL of 0.5% bupivacaine + 0.5 mL sterile saline
- Label with drug, concentration, and expiration date
- See “IACUC Guidelines: Use of Drugs and Chemicals in Laboratory Animals” for further details (proper handling, expiration guidance, etc.)
- 50/50 Mixture of Lidocaine/Bupivacaine
- Dilute 2% (20 mg/ml) Lidocaine 1:4 and 0.5% (5mg/mL) Bupivacaine 1:2 in the same vial
- Example: 0.5 mL 2% lidocaine + 1 mL 0.5% bupivacaine + 0.5 mL sterile saline
- Label with drug, concentration, and expiration date
- See “IACUC Guidelines: Use of Drugs and Chemicals in Laboratory Animals” for further details (proper handling, expiration guidance, etc.)
Maximum Volumes
|
Weight of Mouse |
Maximum Volume Diluted Lidocaine (0.5%) or Mixture Do not exceed: |
Maximum Volume Diluted Bupivicaine (0.25%) Do not exceed: |
|---|---|---|
|
25g |
0.03ml |
0.08ml |
|
35g |
0.05ml |
0.11ml |
|
45g |
0.06ml |
0.14ml |
|
55g |
0.07ml |
0.17ml |
|
Weight of Rat |
Maximum Volume Diluted Lidocaine (0.5%) Do not exceed: |
Maximum Volume Diluted Bupivicaine (0.25%) Do not exceed: |
|---|---|---|
|
250g |
0.35ml |
0.8ml |
|
350g |
0.49ml |
1.12ml |
|
250g |
0.63ml |
1.44ml |
|
550g |
0.77ml |
1.76ml |
Line Block Procedure:
- Determine maximum dose that can be used depending on weight of animal
- Anesthetize the animal, and prepare skin for aseptic surgery
- Inject local anesthetic into the subcutaneous space (“line block”) below the planned incision line, while withdrawing needle along incision line
Local Anesthetics should be used pre-operatively (before the first incision) and can be used in conjunction with opioid analgesics and/or NSAIDs for controlling moderate to severe pain. Intramuscular and intravenous injection of local anesthetics must be avoided. Systemic toxicity (seizures, heart rhythm disturbances and death) results from overdose or accidental intravenous injection.
Common Analgesic Agents
The following is a list of commonly used analgesic agents by species. This list is not inclusive; other analgesic agents may be listed and used in an Animal Protocol.
Other accepted resources for appropriate analgesics include the following formularies:
- Plumb’s Veterinary Drug Handbook (Plumb)
- Anesthesia and Analgesia in Laboratory Animals (ACLAM)
- Formulary for Laboratory Animals (Hawk)
- Exotic Animal Formulary (Carpenter & Marion)
- Swine in the Laboratory (Swindle)
- Sheep and Goat Medicine (Pew)
Appropriate analgesic drugs and dosage(s) should be determined in consultation with an OAR or IACUC veterinarian.
Mouse analgesics:
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
See above |
|
NSAID |
Flunixin meglumine |
2.5 mg/kg SC every 12-24 hours |
|
NSAID |
Meloxicam /Meloxicam-SR* |
1-5 mg/kg SC every 24 hours |
|
NSAID |
Carprofen |
5 mg/kg SC every 24 hours |
|
Opioid |
Buprenorphine |
0.05-2.5 mg/kg SC or IP every 6-8 hours |
|
Opioid |
Buprenorphine ER-LAB (sustained release)** |
0.5-2.0 mg/kg SC every 48 hours |
|
Opioid |
Butorphanol |
0.2-2 mg/kg SC or IP every 2-4 hours |
|
Opioid |
Oxymorphone |
0.2-0.5 mg/kg SC every 6-12 hours |
*Meloxicam-SR is a new product which claims 72 hours of duration in cats and dogs. Independent studies have not demonstrated efficacy beyond 24 hours post-administration in rodents. Use of this product in rodents is under continuing review by OAR veterinarians and requires close monitoring for signs of pain if expected effect is greater than 24 hours.
**Contact OAR Veterinarians for prescription and training
Rat analgesics:
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
See above |
|
NSAID |
Ketoprofen |
5 mg/kg SC or PO every 24 hours |
|
NSAID |
Meloxicam |
1-2 mg/kg SC or PO every 24 hours |
|
NSAID |
Carprofen |
5 mg/kg SC every 24 hours |
|
Opioid |
Buprenorphine |
0.02-0.5 mg/kg SC, IV or IP every 6-8 hours |
|
Opioid |
Buprenorphine ER-LAB (sustained release)** |
1.0-1.2 mg/kg SC every 48 hours |
|
Opioid |
Butorphanol |
0.2-2 mg/kg SC or IP every 2-4 hours |
|
Opioid |
Oxymorphone |
0.2-0.5 mg/kg SC every 6-12 hours |
*Meloxicam-SR is a new product which claims 72 hours of duration in cats and dogs. Independent studies have not demonstrated efficacy beyond 24 hours post-administration in rodents. Use of this product in rodents is under continuing review by OAR veterinarians and requires close monitoring for signs of pain if expected effect is greater than 24 hours.
**Contact OAR Veterinarians for prescription and training
Rabbit Analgesics
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
Line block, consult with vet staff |
|
NSAID |
Carprofen |
1-2.2 mg/kg PO every 12 hours |
|
NSAID |
Flunixin meglumine |
1-2 mg/kg SC or IM every 12-24 hours |
|
NSAID |
Ketoprofen |
3 mg/kg SC every 24 hours |
|
NSAID |
Meloxicam |
0.2-0.6 mg/kg SC or PO every 24 hours |
|
Opioid |
Buprenorphine |
0.01-0.05 mg/kg SC, IM or IV every 6-12 hours |
|
Opioid |
Buprenorphine ER (sustained release)** |
0.1-0.3 mg/kg SC every 48-72 hours |
|
Opioid |
Butorphanol |
0.1-1 mg/kg SC, IM or IV every 4-6 hours |
|
Opioid |
Oxymorphone |
0.05-0.2 mg/kg SC or IM every 8-12hours |
**Contact OAR Veterinarians for prescription and training
Pig Analgesics
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
Line block, consult with vet staff |
|
NSAID |
Carprofen |
2-3 mg/kg IM, SC or PO every 24 hours |
|
NSAID |
Flunixin meglumine |
1-4 mg/kg IM or SC every 12-24 hours |
|
NSAID |
Ketoprofen |
1-3 mg/kg IM, SC or PO every 24 hours |
|
NSAID |
Meloxicam |
0.4 mg/kg SC every 24 hours |
|
NSAID |
Phenylbutazone |
4-8 mg/kg PO every 12 hours |
|
Opioid |
Buprenorphine |
0.005-0.1 mg/kg IM, SC or IV every 8-12 hours 0.005-0.01 recommended for augmenting anesthesia; 0.01-0.1 recommended for post-operative pain control |
|
Opioid |
Buprenorphine ER (sustained release)** |
0.12-0.2 mg/kg SC every 48-72 hours |
|
Opioid |
Butorphanol |
0.1-0.3 mg/kg IM, SC or IV every 8-12 hours |
| Opioid | Oxymorphone | 0.15 mg/kg IM or SC every 4 hours |
Sheep Analgesics
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
Line block, consult with vet staff |
|
NSAID |
Carprofen |
4 mg/kg SC every 24 hours |
|
NSAID |
Flunixin meglumine |
1-2 mg/kg IM, IV or PO every 12-24 hours |
|
Opioid |
Ketoprofen |
2-3 mg/kg IM, IV or PO every 24 hours |
|
Opioid |
Phenylbutazone |
2-6 mg/kg IV or PO every 12 hours |
|
Opioid |
Buprenorphine ER (sustained release)** |
0.05-0.3 mg/kg SC or IM every 48-72 hours |
|
Opioid |
Buprenorphine |
0.005-0.01 mg/kg IM every 4-6 hours |
|
Opioid |
Butorphanol |
0.5 mg/kg SC every 2-3 hours |
**Contact OAR Veterinarians for prescription and training
Ferret Analgesics
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
Local |
Bupivacaine 0.25% Lidocaine 0.5% |
Line block, consult with vet staff |
|
NSAID |
Carprofen |
1 mg/kg PO every 12-24 hours |
|
NSAID |
Flunixin meglumine |
0.3-2 mg/kg SC every 12-24 hours |
|
NSAID |
Ketoprofen |
1 mg/kg PO, SC or IM every 24 hours |
|
Opioid |
Buprenorphine |
0.01-0.03 mg/kg SC, IM or IV every 8-12 hours |
|
Opioid |
Butorphanol |
0.05-0.5 mg/kg SC, IM or IV every 8-12 hours |
|
Opioid |
Oxymorphone |
0.05-0.2 mg/kg SC, IM or IV every 8-12 hours |
Aquatic Species:
There are currently no pharmacokinetically based recommendations regarding efficacious drug dosing of analgesics that can be safely administered to Xenopus frogs, Danio fish, or many other aquatic animals. Limited lethality data suggest narrow safety indices for semi-terrestrial species such as the bullfrog. In fully aquatic species, analgesic agents with sedating qualities (e.g. opioids) carry the risk of drowning due to over sedation. Analgesic drugs and doses should be chosen and used very carefully. Consultation with an IACUC or OAR veterinarian prior to administration of analgesic agents and doses is required.
Some published analgesic doses for various amphibian species include:
|
Class |
Agent |
Dose/Route/Frequency |
|---|---|---|
|
NSAID |
Flunixin meglumine |
25 mg/kg intraceolomic (q24-48 hours) |
|
Opioid |
Buprenorphine |
14 mg/kg into dorsal lymph sac (duration variable) OR 38 mg/kg SQ (duration >4 hours in leopard frogs) |
|
Opioid |
Butorphanol |
25 mg/kg intraceolomic q12 hours |
|
Alpha agonist |
Dexmedetomidine |
120 mg/kg dorsal lymph sac q24 hours |
|
Alpha agonist |
Xylazine |
10 mg/kg intracoelomic q12-24 hours |
Non-pharmacologic methods of pain management
When pharmacological intervention (i.e. analgesic agents) is not possible or in addition to pharmacological intervention, these methods can be employed to decrease pain:
- Skilled surgeon (reduce unintentional surgical trauma)
- Acclimation of animals prior to surgery
- Enhance environment to minimize stress (soft bedding, easy food access, soft food, warm temperature, decrease human traffic, decrease noise)
- Fluid therapy to sustain hydration
Last Reviewed by the IACUC 7/17/2023
Analgesia - Buprenorphine ER (Informational Sheet)
Informational Sheet: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. Informational Sheets provide information about frequently asked questions and represents guidance for best practices. Deviation from the recommendation(s) does not require specific justification.
Purpose: The purpose of this document is to provide labs with adequate information needed to add Buprenorphine Extended-Release (formerly) Sustained-Release to an animal protocol, obtain a prescription, order, store, and use the drug for research animals. It is also to provide labs with information about extended-release buprenorphine options, including the use of FDA-approved versus compounded versions.
Buprenorphine Extended-Release (Bup ER) is a patented compounded opioid that provides up to 48 hours of analgesia and is only available by prescription from a veterinarian. See the IACUC Guidelines on Analgesia for dosing information.
How to add Bup ER to an animal protocol:
If Buprenorphine ER is the only DEA controlled drug that you will be using on your protocol, you will need to answer the DEA controlled substance question in the Special Circumstances and Hazards section as “YES”. You will be prompted to name the person whose license you will be using. Because this is a prescription, you are not required to have a DEA license; however this question must be answered to complete and submit your protocol. Please list ‘OAR Veterinarian’ as the license holder in this case.
If you are using other DEA controlled substances as well as Buprenorphine ER, no additional information is required as you will answer YES and provide the license holder for use of those other substances.
How to obtain a prescription for Bup ER:
Go to the Drug Order [1] section of the OAR website and click ‘Buprenorphine-ER Prescription Request Form’, which processes your request through Workflow using your HawkID. Fill out the information as accurately as possible, using the HawkID of the Principal Investigator for the protocol. It may take up to 7 business days for your prescription to be submitted to the manufacturer.
How to order Bup ER:
Once you receive confirmation that your prescription has been submitted, contact the manufacturer Wedgewood Pharmacy (formerly ZooPharm) to set up an account and/or place your order. Wedgewood Pharmacy may be contacted by phone (877-357-6613) or on the website (https://www.wedgewoodpharmacy.com/contact-us.html [2]). If refills are available for your prescription and you already have an account, log-in to your account to order. Bup ER costs $150-190 per 5cc/ml vial, depending upon concentration, and shipping is approximately $35. One vial is sufficient for approximately 80 mice or 16 rats.
Storage of Bup ER:
Bup ER is shelf stable until the labeled expiration (typically 6 months to 1 year after compounding) unless you are otherwise informed by your veterinarian. The label may state a recommendation for disposal after the seal is punctured. Research labs are not required to adhere to this recommendation. While prudent to keep Bup ER locked up for safety and security reasons, as a prescription drug it is not required that you do so. If you do however lock it up, do not store it in the same lock box as other DEA controlled drugs. You will need a different storage location such as a locked drawer or cabinet.
Use of Bup ER:
- Do NOT dilute Bup-ER as it affects the rate of release. Dilution of Bup-ER is not permitted.
- MUST be given as a subcutaneous injection
- Bup-ER is viscous and may require larger needle sizes (22-23 gauge) for administration and may most easily be administered under anesthesia.
- Consult with an OAR Veterinarian regarding concerns of dosing and administration
- Administration of small volumes may be aided by drawing up air into the syringe prior to the dose or using low-dead space syringes.
Recent FDA guidance GFI #256 Compounding Animal Drugs from Bulk Drug Substances has been implemented that impacts the use of Buprenorphine ER (formerly Buprenorphine SR). Per the guidance, compounded drugs that have an FDA-approved equivalent must have a medical rationale describing the clinical difference between them. This indicates that justification must be provided in order to procure Bup ER as there is an FDA-approved extended-release buprenorphine product for mice and rats called Ethiqa XR.
All labs should review their use of extended-release buprenorphine and consider use of the FDA-approved Ethiqa XR for future studies. For further prescriptions of Buprenorphine ER/SR, a medical rationale will be required and reviewed by the prescribing veterinarian, who will make final decision to write the prescription.
Below is a chart comparing the two Buprenorphine extended-release products:
|
Drug details |
Ethiqa XR |
Buprenorphine ER/SR |
|---|---|---|
|
FDA Approval Status |
FDA-Approved |
Compounded
|
|
Analgesia Length |
Up to 72 hrs |
48-72 hrs |
|
Dosage |
Mouse: 3.25 mg/kg SQ Rat: 0.65 mg/kg SQ |
Mouse: 0.5-2 mg/kg SQ Rat: 1-1.2 mg/kg SQ |
|
Concentration |
1.3 mg/mL |
0.5, 1, and 3 mg/mL |
|
Volume |
3 mL |
5 mL |
|
Shelf Life Unopened |
24 months |
Varies by lot; typically 10-12 months from date of purchase |
|
Shelf Life after Piercing Rubber Seal |
90 days |
30 days |
|
Cost per vial* |
$415 |
$150-190 |
|
How to Obtain |
Investigators can purchase on their own with their individual DEA license through multiple suppliers/distributors |
Prescription must be written by a veterinarian and drug ordered through Wedgewood Pharmacy |
* As of 6/9/23. Price subject to change.
Please note that Ethiqa XR is considered a controlled substance and since it is acquired directly by the researcher, is managed as any other controlled substance in regards to storage, inventory, and disposal.
More information about Ethiqa XR, including suppliers/distributors, can be found here: https://ethiqaxr.com/ [3]
Last Reviewed by the IACUC 10/11/2023
Anesthesia (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to provide guidance on commonly used inhaled and injectable anesthetic agents for use in animal research at the University of Iowa. All anesthetic agents used in animals must be listed on an approved Animal Protocol. All anesthetic procedures must be performed by appropriately trained personnel.
Recordkeeping for Anesthesia/Sedation
- Applies to all survival and non-survival procedures performed under anesthesia
- Mice and Rats (as well as fish, amphibians, reptiles, and birds)
- Brief procedures using inhalant/absorbed anesthetics
- No anesthesia record is required if anesthesia is less than 5 minutes.
- Use of eye lubrication is not required for anesthesia procedures lasting less than 5 minutes but is strongly recommended.
- Brief procedures are those that cause no more than momentary pain (e.g. blood collection, tail vein injection, tail snip for genotyping or intranasal inoculations, fin snip on zebrafish)
- No anesthesia record is required if anesthesia is less than 5 minutes.
- For all other procedures not described above
- An anesthesia record is required.
- Eye lubrication is required.
- Animals should be monitored until full recovery if survival or euthanasia if non-survival.
- Document the following:
- Date
- Principal Investigator
- Animal Protocol number
- Animal ID
- Species
- Weight
- Procedure
- Agent(s) used, dosage, route of administration
- Time of induction of anesthesia
- Time of recovery from anesthesia or time of euthanasia
- Brief procedures using inhalant/absorbed anesthetics
- USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
- Any anesthetic event
- An individual record for each animal is required
- Document the following:
- Date
- Principal Investigator
- Animal Protocol number
- Animal ID
- Species
- Weight
- Procedure
- Agent(s) used, dosage, route of administration
- Time of induction of anesthesia
- Time of recovery from anesthesia or time of euthanasia
- Monitoring (every 15 minutes until recovery or euthanasia)
- Any anesthetic event
- Please see the surgery guidelines for surgical record information
- Click here for template anesthesia records [4]
Supportive Care of Animals During Anesthesia
- Apply ophthalmic ointment to both eyes to prevent desiccation for any anesthesia longer than 5 minutes.
- Rodents can quickly become hypothermic under anesthesia that is longer than 5 minutes and heat support is required. Maintain normal body temperature using an allowed heating support option (below).
- Provide fluids (e.g., IV, IP, SQ) to animals during prolonged anesthesia to maintain adequate hydration as described in the approved Animal Protocol
Allowed Heating Support Options:
- Warm circulating water blanket, thermal pads, and/or warmed IV fluids.
- Electric heating pads must have temperature setting capability and digital readout. Recommended heating pad options include but are not limited to the following:
- Kent Scientific Far Infrared Warming
- Parkland Scientific Lab Animal Warm Water Blankets and Circulators
- Conduct Science Heating Pads
- Braintree scientific Delta Phase Isothermal Pads and Insulators
- Please contact OAR Veterinary Care Staff to review alternative heating pad options.
Prohibited Heating Support Options:
- DO NOT use an “over the counter” low/medium high setting electric heating pad as these are prone to overheating
- Common Brands: Sunbeam, Pure Relief, Geniani, etc.
Monitoring and Assessment of Anesthesia (while procedure is being performed)
- USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
- Monitor heart rate, respiratory rate, and temperature
- Document these parameters at least every 15 minutes during anesthesia
- For rodent species, qualitative monitoring for normal cardiovascular and respiratory function may be sufficient, as it is difficult to assess these parameters quantitatively.
- For all other species, quantitative monitoring (numerical heart and respiratory rates) is expected.
- If using a ventilator, note ventilation rate and tidal volume in the records
- Monitor hemodynamic parameters to assure adequate gas exchange
- Mucous membranes should be pink and moist
- Capillary refill time should be less than 2 seconds
- Monitoring for at least one indicator of deep pain recognition (pedal reflex, pinna reflex, etc.) should be performed regularly to ensure adequate anesthesia
- Adjust the depth of anesthesia as dictated by changes in the monitored parameters to ensure continued surgical plane of anesthesia
- Monitor heart rate, respiratory rate, and temperature
- Mice and Rats
- Monitor respiratory rate and effort, color of mucous membranes, and reflected eye color (in albino animals) at regular intervals (no longer than 15-minute intervals)
- Assess level of anesthesia by pedal reflex (firm toe pinch) and adjust anesthetic delivery as appropriate to maintain surgical plane
Anesthetic Recovery
- Large Animals
- Food and water bowls must be removed from the recovery cage
- Monitor each animal continuously until holding sternal position:
- Maintaining sternal recumbency (lying upright on chest)
- Heart and respiratory rate
- Body temperature
- A circulating warm water heating pad is recommended
- Hydration as assessed by skin turgor or mucous membrane “tackiness”
- Monitor each animal at least every 15 minutes until ambulatory (walking normally)
- Deflate and remove the intubation tube once animal can swallow
- Do not leave animal unattended while intubation tube is in place
- Occasionally reposition recumbent animals to promote a quicker recovery
- Animals should not remain with the same side down for more than four (4) hours
- Lower animal’s head slightly below chest level to prevent aspiration if vomiting occurs
- Mice and Rats
- Place rodent in warm, clean, dry, quiet environment away from other animals
- Cover or replace bedding material with toweling material
- Bedding can stick to eyes or be inhaled while animals are recovering from anesthesia
- Provide warmth during recovery (required if anesthesia is greater than 5 minutes). Maintain normal body temperature using:
- An allowed heating support option (see above). *Note, when using an allowed heating pad option during anesthesia recovery, place cages so that approximately 50% of the cage is on the allowed heating pad option. This allows the animal to move away from the heat as it recovers from anesthesia.
- Incandescent lamp (50-75 watt) 12-14 inches away from rodent
- Position lamp so that rodent can escape the light sources if desired
- Attentive monitoring must be performed to prevent overheating of rodent
- Use of a temperature-controlled cage/incubator
- If listed in approved Animal Protocol, warm sterile saline can be administered to replace body fluids lost during surgery
- All animals must be continuously visually monitored (i.e. present in the room with ability to see the animal) until maintaining upright posture and walking normally about the cage before completion of monitoring and return to the animal housing room. Physiological parameters must be monitored at least every 15 minutes as noted above.
Anesthetic Agents
The following is a list of commonly used anesthetic agents. This list is not inclusive; other anesthetic agents may be listed and used in an Animal Protocol.
Other accepted resources for appropriate analgesics include the following formularies:
- Plumb’s Veterinary Drug Handbook (Plumb)
- Anesthesia and Analgesia in Laboratory Animals (ACLAM)
- Formulary for Laboratory Animals (Hawk)
- Exotic Animal Formulary (Carpenter & Marion)
- Handbook of Veterinary Anesthesia (Muir & Hubbell)
- Swine in the Laboratory (Swindle)
Appropriate anesthetic drugs and dosage(s) should be determined in consultation with an OAR or IACUC veterinarian.
Inhaled Anesthetic Agents
ISOFLURANE/SEVOFLURANE - VAPORIZER
- Isoflurane/sevoflurane must be administered with a properly calibrated vaporizer when used as an anesthetic agent for surgery
- Anesthetic gases must be scavenged properly
- Direct exhaust
- Activated charcoal canister (e.g. F/Air canister)
- Should be weighed before each use and must be discarded when maximum weight is achieved
- Weight records must be maintained
- Vaporizers must be calibrated at least yearly
- Calibration records for each vaporizer must be maintained
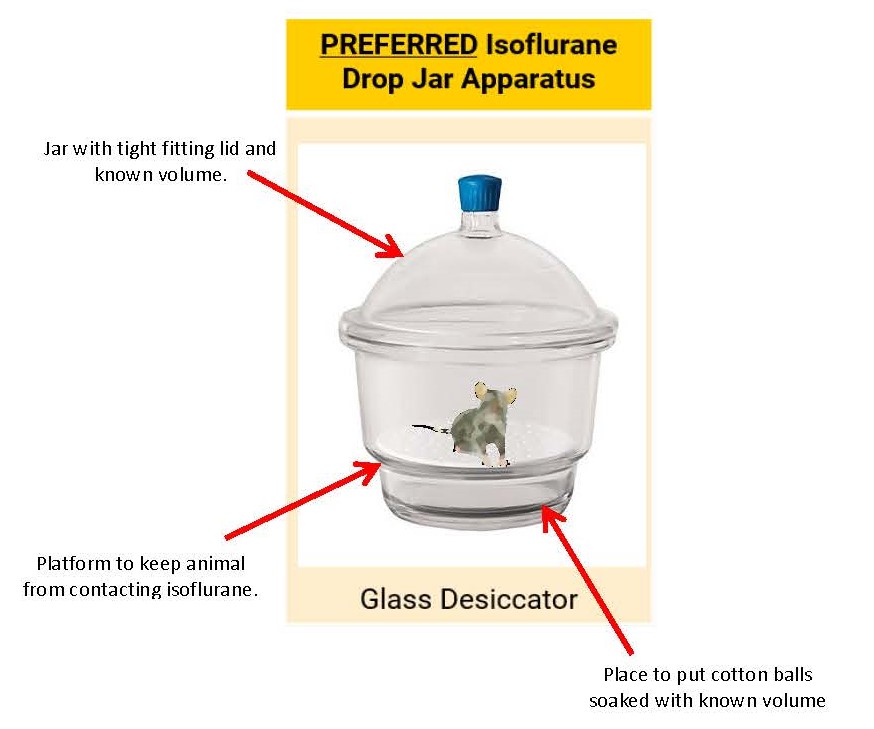
ISOFLURANE – DROP JAR METHOD
- NOT for sevoflurane use; the concentration of sevoflurane cannot be accurately controlled with the drop jar method.
- Ensure that use is approved in the Animal Protocol.
- Isoflurane can be administered in an anesthetic drop jar for a single, brief procedure
- Cannot be used for any surgical procedures (includes non-survival and survival surgeries) or non-brief methods of euthanasia (e.g. perfusion)
- Anesthetic gases must be scavenged properly
- Fume hood
- Hard-ducted biosafety cabinet
- Animals must be clearly visible within the container.
- Animals must not come into direct contact with liquid isoflurane
- Anesthetize one animal at a time.
- Drop jar must be cleaned (i.e. removal of urine/feces) between animals with appropriate disinfectants.
- 70% EtOH is a good cleaner but is a weak disinfectant.
- It is required that labs use a strong disinfectant for cleaning animal use surfaces. (see examples of appropriate hard surface disinfectants here [5].)
- Use of this method with a 50 mL conical tube is prohibited.

Drop jar dosing for Isoflurane: Internal Volume of Chamber (L) and isoflurane liquid required (mL)
| Isoflurane Concentration achieved | 1L | 2L | 3L | 4L | 5L |
|---|---|---|---|---|---|
| 1% | 0.05mL | 0.10 mL | 0.15 mL | 0.20 mL | 0.26 mL |
| 2% | 0.10 mL | 0.20 mL | 0.31 mL | 0.41 mL | 0.51 mL |
| 3% | 0.15 mL | 0.31 mL | 0.46 mL | 0.61 mL | 0.77 mL |
| 4% | 0.20 mL | 0.41 mL | 0.61 mL | 0.82 mL | 1.02 mL |
| 5% | 0.26 mL | 0.51 mL | 0.77 mL | 1.02 mL | 1.28 mL |
75% CO2 / 25% O2
- 75% CO2 / 25% O2 can be used for single, brief procedures
- Cannot be used for any surgical procedures (includes non-survival and survival surgeries).
- Available pre-mixed from vendors
- Use precaution; animals can be easily overdosed
- Not intended for euthanasia
- Contact a member of the Office of Animal Resources veterinary staff if you are unfamiliar with the proper use of CO2/O2
Absorbed Anesthetic Agents
- Tricaine Methanesulfonate (MS-222)
- Frogs:
- 0.05-0.2% (500-2000mg/L) solution
- Solution must be buffered with sodium bicarbonate to a pH of 7.0-7.5
- Immerse frog in solution for 10-20 minutes
- Level of anesthesia is judged by loss of righting reflexes, loss of gulping reflex and loss of withdrawal response to toe pinch
- Fish:
- 0.0025-0.01% (25-100mg/L) solution
- Solution must be buffered with sodium bicarbonate to a pH of 7.0-7.5
- Immerse fish in solution until appropriate anesthetic depth is observed
- Level of anesthesia is judged by loss of equilibrium, loss of response to noxious stimuli (pinching base of tail), rate of opercular movement and gill color
- Storage
- Tricaine (liquid solution) can be stored at room temperature for 3-5 days if protected from exposure to light.
- Tricaine (liquid solution) can be stored at 4°C (i.e. – the refrigerator) for 1 month if stored if protected from light.
- Tricaine (liquid solution) can be stored at -20°C (i.e. – the freezer) for 1 year if protected from the light.
- Tricaine (powder) can be stored at room temperature for up to 5 years if stored in a dark container.
- Frogs:
Injectable Anesthetic Agents
COMMONLY USED INJECTABLE ANESTHETIC AGENTS
MOUSE
|
Agent |
Dosage |
Duration of anesthesia |
|---|---|---|
|
Ketamine/xylazine* |
ketamine 80-100 mg/kg IP xylazine 10-12.5 mg/kg IP |
20-30 minutes |
|
Ketamine/xylazine cocktail* |
KX mouse cocktail 0.1mL/20g mouse wt. IP Contains: 87.5 mg/kg Ketamine 12.5 mg/kg Xylazine |
20-30 minutes |
|
Ketamine/xylazine/acepromazine |
ketamine 60-100 mg/kg IP xylazine 10-15 mg/kg IP acepromazine 2-5 mg/kg IP |
60-90 minutes |
|
Pentobarbital |
50 mg/kg IP |
20-40 minutes |
|
Avertinǂ See warning below |
240 mg/kg IP |
30 minutes |
*Ketamine/xylazine without combination with an analgesic agent (opioid or NSAID) may be insufficient to produce a surgical plane of anesthesia. Administration of appropriate analgesic agents prior to surgery and/or addition of acepromazine will augment the anesthetic effect of ketamine/xylazine.
** Preparation instructions for the ketamine/xylazine cocktail may be found below.
ǂ WARNING: NIH and European guidelines discourage the use of Avertin. Preparation and storage requirements for Avertin may be found below.
* GUIDELINES - PREPARATION OF KETAMINE/XYLAZINE COCKTAIL FOR MICE
- Use of a sterile injection vial is required (e.g. redtop blood collection tube; commercial injection vial)
- Mixing instructions:
- Verify the concentration of your drugs prior to mixing
- For a 10mL vial using ketamine 100 mg/mL and xylazine 100 mg/mL add:
- 1.75mL ketamine (100 mg/mL)
- 0.25 mL xylazine (100 mg/mL)
- 8 mL saline or sterile water for injection
- Use of the following template for a label is recommended:
- Mouse Anesthetic Mix: Ketamine/Xylazine
- Dosage: 0.1 ml/ 20gm IP
- Delivers: 87.5 mg/kg Ketamine/12.5 mg/kg Xylazine
- Concentration: 17.5 mg/mL Ketamine/2.5 mg/mL Xylazine
- Expires: ____________
-
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
- E.g.: Diluted on 8/13/21, ketamine expires 12/10/2022, xylazine expires 10/10/21 and sterile water for injection expires 1/12/2023; the expiration date for the cocktail is 10/10/21
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
RAT
|
Agent |
Dosage |
Duration of anesthesia |
|---|---|---|
|
Ketamine/xylazine |
ketamine 40-100 mg/kg IP xylazine 5-13 mg/kg IP |
60-80 minutes |
|
Ketamine/xylazine cocktail*
|
KX rat cocktail 0.1 mL/100g rat wt. IP Contains: 91 mg/kg Ketamine 9.1 mg/kg Xylazine |
60-80 minutes |
|
Ketamine/xylazine/acepromazine |
ketamine 20-50 mg/kg IP xylazine 2-10 mg/kg IP acepromazine 0.5-1.5 mg/kg IP |
60-120 minutes |
|
Pentobarbital |
30-50 mg/kg IP |
90-120 minutes |
*Ketamine/xylazine without combination with an analgesic agent (opioid or NSAID) may be insufficient to produce a surgical plane of anesthesia. Administration of appropriate analgesic agents prior to surgery and/or addition of acepromazine will augment the anesthetic effect of ketamine/xylazine.
** Preparation instructions for the ketamine/xylazine cocktail may be found below.
GUIDELINES - PREPARATION OF KETAMINE/XYLAZINE COCKTAIL FOR RATS
- Use of a sterile injection vial is required (e.g. redtop blood collection tube; commercial injection vial)
- Mixing instructions:
- Verify the concentration of your drugs prior to mixing
- For a 10mL vial using ketamine 100 mg/mL and xylazine 100 mg/mL add:
- 10 mL ketamine (100 mg/mL)
- 1 mL xylazine (100 mg/mL)
- Use of the following template for a label is recommended:
- Rat Anesthetic Mix: Ketamine/Xylazine
- Dosage: 0.1 ml/ 100gm IP
- Delivers: 91 mg/kg Ketamine, 9.1 mg/kg Xylazine
- Concentration: 91 mg/mL Ketamine, 9.1 mg/mL Xylazine
- Expires: ____________
-
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
- E.g.: Diluted on 8/13/21, ketamine expires 12/10/2022, xylazine expires 10/10/21 and sterile water for interjection expires 1/12/2023, the expiration date for the cocktail is 10/10/21
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
RABBIT
| Agent | Dosage |
|---|---|
| Ketamine/xylazine |
ketamine 22-50 mg/kg IM xylazine 2.5-10 mg/kg IM |
| Pentobarbital | 20-60 mg/kg IV |
PIG
| Agent | Dosage |
|---|---|
| ketamine/xylazine |
ketamine 20 mg/kg IM xylazine 2 mg/kg IM |
| Telazol/ketamine |
telazol 4.4 mg/kg ketamine 2.2 mg/kg |
| Pentobarbital | 20-40 mg/kg IV |
SHEEP
| Agent | Dosage |
|---|---|
| Ketamine/xylazine |
5-15 mg/kg IM ketamine 0.05-0.2 mg/kg IM xylazine |
| Thiopental | 10-16 mg/kg IV |
FERRET
| Agent | Dosage |
|---|---|
| Ketamine/xylazine |
10-25 mg/kg IM ketamine 0.25-0.5 mg/kg IM xylazine |
Other species or anesthetic agents:
Please contact a University of Iowa clinical veterinarian [6] for consultation
GUIDELINES FOR PREPARATION AND STORAGE OF AVERTIN (TRIBROMOETHANOL)
- Avertin is a quick-acting, non-pharmaceutical grade* anesthetic that is used for short duration surgical procedures in mice.
- NOTE: Per the Guide for the Care and Use of Laboratory Animals 8th edition, the use of non-pharmaceutical grade chemicals or substances needs to be described and scientifically justified in the Animal Protocol.
- Precautions:
- Do not administer Avertin if you have a/an:
- Non-sterile solutions
- Outdated solutions
- More concentrated solutions
- Higher dosages than recommended
- Avertin should only be administered one time (no redosing) due to resultant gastrointestinal irritation
- Do not administer Avertin if you have a/an:
- Disadvantages of the use of Avertin:
- Tissue irritation, especially at high dosages, high concentrations or repeated doses
- Degrades in the presence of heat or light to produce toxic byproducts which can be both nephrotoxic and hepatotoxic
- Can cause intestinal ileus several weeks after injection
- Unpredictable effects in mice under 16 days of age or in mice with altered carbohydrate metabolism (e.g., mouse strains used as diabetes or obesity models)
- Some European journals are rejecting research manuscripts when Avertin is used as an anesthetic
- Ingredients:
- 2.5 grams 2,2,2 Tribromoethanol
- 5 ml 2-methy-2-butanol (amylene hydrate, tertiary amyl alcohol)
- 200 ml distilled water - neutral pH (sterile)
- Preparation (12.5 mg/ml solution):
- Dissolve 2.5 grams Tribromoethanol in 5 ml amylene hydrate.
- Heat dissolved solution to 40°C while stirring vigorously.
- Do not exceed 40°C.
- Add distilled water, stirring continuously, up to a final volume of 200 ml.
- Filter sterilize through a Millipore filter (0.5 micron)
- Storage:
- Filter final solution into red-cap blood collection tubes or amber (brown) colored sterile glass containers
- Solution container must be wrapped in aluminum foil to protect solution from light
- Solution container must be labeled with contents and date of preparation
- Store in refrigerator or freezer
- Expiration has occurred if any one of the following conditions are met:
- Two week expiration date if stored in refrigerator
- One year expiration date if stored in freezer
- Crystallization of solution
- Solution has turned yellow in color
Last Reviewed by the IACUC 5/10/2023
Anesthesia Monitoring Templates
Rodent Anesthesia Monitoring:
Word (editable): ![]() Rodent Non-surgical Anesthesia Monitoring Template.docx [7]
Rodent Non-surgical Anesthesia Monitoring Template.docx [7]
Word (editable): ![]() Rodent Surgical Monitoring Template.docx [8]
Rodent Surgical Monitoring Template.docx [8]
USDA Covered Species Anesthesia Monitoring:
Nonsurgical Procedures:
Word (editable): ![]() USDA species Nonsurgical Anesthesia Monitoring Template.docx [9]
USDA species Nonsurgical Anesthesia Monitoring Template.docx [9]
Surgical Procedures:
Biologic Testing - Guidance and Procedures for Rodent Biologic Testing (Informational Sheet)
Informational Sheet: The Office of Animal Resources has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. This Informational Sheet provides the current guidance on recommended testing of research biologics for pathogens.
Background:
Administration of human or animal tissues or other biological materials into animals, especially rodents, is a common research practice. These materials may be contaminated with a variety of agents that may be infectious to humans or animals and potentially jeopardize the health of both. Additionally, contaminating pathogens may act as a confounding variable on research results. Collaborative research is the rule rather than the exception in today’s research environment. As rodents, especially mice, and biological materials including cell lines are shared between investigators within and between institutions, it greatly facilitates collaborative research to ensure that animals and biological samples are free of pathogens.
Definition:
- Rodent Biologic Material:
- Cultured or primary cells, tissues, infectious agents, or serum/plasma that originates in a rodent species (mouse, rat, or other rodent)
- Cells, tissues, infectious agents, or serum/plasma from humans and other non-rodent animals which have been exposed to rodents or rodent biologics either directly (in vivo passage) or indirectly (via tissue culture or treatment)
Recommendations for Testing:
Rodent colonies within the Animal Facilities at the University of Iowa are screened through a health monitoring program for infectious diseases and are maintained free of viruses and other microbial agents capable of interfering with research. The health of the colonies and the integrity of research can be endangered by inadvertent introduction of untested biological material carrying pathogens.
It is recommended that Rodent Biologic Materials be demonstrated free of common rodent pathogens prior to administration to a rodent housed in OAR Animal Housing.
Testing Procedures and Resources:
- Rodent Biologic Materials may be demonstrated free of excluded pathogens in the following ways:
- Commercial PCR testing of a representative portion of the cell line or tissue carried in culture, or of the pooled biologic material (serum, infectious agent, etc.)
- Documentation from the vendor/supplier of appropriate testing for excluded pathogens (see list below).
- A contracted rate for PCR testing for all UI-excluded pathogens has been established with the diagnostic labs of IDEXX BioAnalytics
- A combination panel of excluded pathogen testing WITH confirmation of cell line identity is also available from IDEXX BioAnalytics, for those who need to perform any cell line confirmation testing.
- Contact a veterinarian at OAR-veterinarian@uiowa.edu [11] to ensure submission at contract rates for these panels.
- Alternately, Charles River Diagnostic Lab’s “Mouse Essential Panel” and/or “Rat Essential Panel” covers all excluded agents and is an accepted option for the required testing. (Details at http://www.criver.com/ [12] under “Health Surveillance” section)
Recommended Agent Panels:
Biologics to be Administered to a Mouse:
|
Agent Name |
Abbreviation(s) |
|---|---|
|
Mouse Hepatitis Virus / Mouse Coronavirus |
MHV |
|
Minute Virus of Mice |
MVM |
|
Mouse Parvoviruses |
MPV1-5 |
|
Mouse Rotavirus/Epizootic Diarrhea of Infant Mice |
MRV/EDIM |
|
Theiler’s Murine Encephalomyelitis Virus |
TMEV |
|
Lymphocytic Choriomeningitis Virus |
LCMV |
|
Sendai virus |
Sendai/Sen |
|
Pneumonia Virus of Mice |
PVM |
|
Reovirus (Type 3) |
Reo3 |
|
Ectromelia Virus (Mousepox) |
ECTRO |
|
Mouse Adenovirus 1 and 2 |
MAD1/2 |
|
Mycoplasma pulmonis |
M. pulmonis |
Biologics to be Administered to a Rat:
|
Agent Name |
Abbreviation(s) |
|---|---|
|
Rat Coronavirus/Sialodacryoadenitis Virus |
RCV/SDAV |
|
Rat Parvovirus |
RPV |
|
Rat Minute Virus |
RMV |
|
Kilham Rat Virus / Rat Virus |
KRV/RV |
|
Toolan’s H-1 Virus |
H-1 |
|
Rat Theilovirus |
RTV |
|
Pneumocystis carinii |
P. carinii |
Lab reviewed by the IACUC 8/14/2024
Blood Collection (Guideline)
Guidelines:The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose:
This document provides direction and guidance on appropriate blood collection methods and volumes for animals used in research at the University of Iowa. These guidelines are intended for use by qualified personnel performing blood collection as described on an IACUC-approved Animal Protocol.
There are several factors to consider when determining the appropriate blood collection volume and technique. These include:
- The species to be sampled
- The size of the animal to be sampled
- The age and health of the animal to be sampled
- The minimum volume required for analysis
- The frequency of sampling necessary
- The training and experience of the personnel performing the collection
- The suitability of sedation and/or anesthesia
The sample volume selected should always be the minimum volume of blood which satisfies experimental needs. Appropriate restraint (physical or chemical) should be employed to minimize risk of injury to the animal and personnel.
Guidelines for calculation of collection volume:
- The maximum permitted blood volume includes blood lost during collection.
- As a general rule, 20 drops = 1 mL (i.e. 5 drops = 250 uL)
Maximal blood collection limits are as follows:
- No more than 1% of the animal’s body weight in one collection or over a 24 hour period
- For example: 25g mouse x 1% = 0.25mL or 250uL maximum blood removal
- No more than 1.5% of the animal’s body weight in two weeks (14 days)
- For example: 200g rat x 1.5% = 3.0mL maximum over 14 days
Frequent Rodent Calculations
Mouse
|
Weight |
Maximum blood loss at one time/ in 24 hours |
Maximum blood loss over 14 days |
|---|---|---|
|
20 g |
200 uL |
300 uL |
|
25 g |
250 uL |
375 uL |
|
30 g |
300 uL |
450 uL |
Rat
|
Weight |
Maximum blood loss at one time/ in 24 hours |
Maximum blood loss over 14 days |
|---|---|---|
|
200 g |
2.0 mL |
3 mL |
|
250 g |
2.5 mL |
3.75 mL |
|
300 g |
3.0 mL |
4.5 mL |
Common Blood Collection Routes By Species
Mouse
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Submandibular vein |
|
|
|
Tail vein* (see below) |
|
|
|
Saphenous vein |
|
|
|
Retro-orbital sinus (see below) |
|
Yes |
|
Cardiac (non-survival) |
|
Yes |
Rat
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Tail vein |
|
|
|
Saphenous vein |
|
|
|
Jugular vein |
Yes |
|
|
Retro-orbital plexus (see below) |
|
Yes |
|
Sublingual vein |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Ferret
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Cephalic vein |
|
|
|
Saphenous vein |
|
|
|
Jugular vein |
Yes |
|
|
Cranial vena cava |
Yes |
|
Rabbit
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Marginal ear vein |
|
|
|
Central auricular artery |
|
|
|
Saphenous vein |
Yes |
|
|
Jugular vein |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Hamsters
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Saphenous vein |
|
|
|
Cephalic vein |
|
|
|
Jugular vein |
Yes |
|
|
Cranial vena cava |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Guinea Pigs
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Ear vein (droplet) |
|
|
|
Saphenous vein |
|
|
|
Cranial vena cava |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Gerbils
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Lateral saphenous vein |
|
|
|
Cranial vena cava |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Xenopus
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Dorsal tarsal vein |
|
Yes |
|
Cardiac (survival) |
|
Yes |
|
Cardiac (non-survival) (also tadpoles) |
|
Yes |
Pigeon
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Brachial wing vein |
Yes |
|
Dog or Cat
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Cephalic vein |
|
|
|
Saphenous vein |
|
|
|
Jugular vein |
|
|
|
Cardiac (non-survival) |
|
Yes |
Pigs
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Ear vein |
Yes |
|
|
Cranial vena cava |
Yes |
|
|
Jugular vein |
Yes |
|
|
Cardiac (non-survival) |
|
Yes |
Ruminants
|
Common Blood Collection Route(s) |
Sedation Recommended |
Anesthesia Required |
|---|---|---|
|
Jugular vein |
|
|
|
Lateral saphenous vein |
|
|
|
Tail vein |
|
|
|
Ear vein |
|
|
Restraint and anesthesia for blood draws:
Restraint methods and anesthesia used to collect blood on research animals must be described and approved in the animal protocol. Examples of restraint devices include rodent restraint tubes, surgical towel or decapicones.
Hemostasis:
Assuring that blood flow has stopped (hemostasis) is of upmost importance after collecting a blood sample. To achieve hemostasis, place gentle pressure over the site of blood collection to stop the bleeding. A gloved hand and a piece of gauze are commonly used. Best practice involves re-inspecting animals approximately 5 minutes after return to their cage to assure blood flow has stopped.
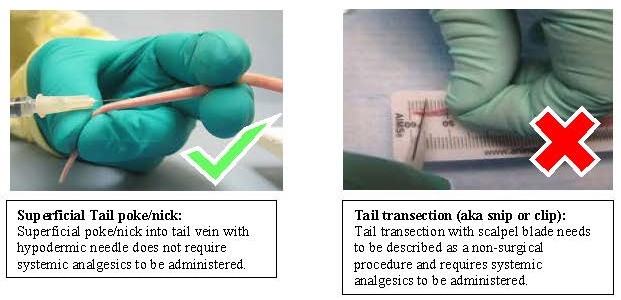
Tail vein collection definitions:
Tail vein collection is defined as use of a hypodermic needle or lancet to access the tail vein along the body of the tail.
Tail transection (also referred to as tail snip or clip) is NOT considered a routine method of blood collection and should be described as a non-surgical procedure with associated monitoring and pain management where appropriate.
- Blood collection of animals greater than 24 days of age
- Use of a systemic analgesic given prior to tail snipping is required.
- systemic analgesic examples: carprofen, meloxicam, buprenorphine, etc
- Analgesics such as lidocaine or bupivacaine are considered local analgesics and when administered alone, do not provide optimal pain management for this procedure.
- Tail snipping for blood collection at this age is potentially painful and should be avoided if possible.
- Use of a systemic analgesic given prior to tail snipping is required.
- More than one sample over the life of the animal:
- Limit of two collections, no more than a total of 2-5mm of the distal tail removed over all collections (including tail snipping for genotyping purposes)
- Use of a systemic analgesic given prior to tail snipping is required.
Techniques for tail vein dilation:
The following techniques may be used to increase blood flow on the tail vein of a mouse or a rat:
1) Use of a heating lamp*
2) Submerging the tail in warm water (no warmer than 40oC/104oF) *
3) Placing rubbing alcohol over the tail
* Animals under a heat lamp must be under direct supervision and care must be exercised to prevent overheating an animal. Animals that overheat may show an increased respiratory rate, decreased movement, red extremities and avoidance of the heat lamp.
Retro-Orbital Sampling:
Retro-orbital blood collection in rodents can provide moderate to large amounts of blood when performed by well-trained personnel. However, severe injuries may occur to the animal if this procedure is not done properly, and available alternatives should be used whenever possible.
The use of retro-orbital bleeding must be described in the protocol and approved by the IACUC. Because rats have a venous plexus rather than a sinus (as in the mouse), the use of this method may result in greater tissue damage and alternative collection sites are strongly recommended.
If retro-orbital collection is necessary, the following guidelines apply:
- General anesthesia is required
- Microhematocrit tubes that hold 50-75 microliters are recommended to minimize risk of injury
- Only one eye may be sampled at any time
- If attempted collection from one eye is unsuccessful, an alternate method approved in the Animal Protocol (e.g. submandibular or saphenous route) must be used, rather than reattempting retro-orbital collection from the same or opposite eye
- Alternate between left and right eyes per session
- No more than 1 collection performed per 7 days (alternate eyes). therefore 14 days between collections in the same eye
- Exception: If repeated sampling within 8 hours is necessary and approved in the Animal Protocol, the retro-orbital sinus may be re-sampled by disrupting the blood clot (from the original collection site) without repeated damage to the sinus, provided the 24 hour maximum blood collection limits are not exceeded
- Please consult with veterinary staff for demonstration and training of proper technique to reduce risk of trauma
- Exception: If repeated sampling within 8 hours is necessary and approved in the Animal Protocol, the retro-orbital sinus may be re-sampled by disrupting the blood clot (from the original collection site) without repeated damage to the sinus, provided the 24 hour maximum blood collection limits are not exceeded
- A maximum of 3 procedures may be performed per eye (up to 6 collections total)
- If injury and/or rupture of the eye or surrounding tissues occurs due to this method, the animal must be immediately euthanized or an OAR veterinarian consulted for guidance
Application of a topical ophthalmic anesthetic during/after collection should be considered to provide post-procedural analgesia.
Last Reviewed by the IACUC 07/17/2023
Breeding - Rodent Breeding Colony Management (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: This policy establishes the parameters for appropriate breeding activities under the University of Iowa animal care and use program. This policy applies to all research personnel which perform breeding activities at the University of Iowa.
Background: The establishment of a rodent breeding colony may be necessary to develop an animal model that is not commercially available, or to produce young animals with specific ages or conditions which cannot be provided by a commercial breeding colony. Investigators developing a new spontaneous or induced mutant animal model might also need to maintain their own breeding colony because there is no alternative source for the animal model.
From a regulatory perspective, tracking all animals utilized in animal research protocols is closely scrutinized by extramural accrediting and oversight bodies. Breeding colonies receive particular attention, and require careful consideration of the justifications for the animal numbers used. Record keeping and colony management practices must demonstrate efforts to utilize animal subjects in ways that conserve genetic traits and are not wasteful.
Maintenance of unnecessary breeding activities increases opportunities for infectious disease entry and transmission, genetic drift of inbred lines, reduces housing space for needed research activities, and increases expense to all investigators due to unrecovered costs of breeding activities. Investigators maintaining colonies exclusively to preserve a genetic line of rodents should consider other conservation strategies such as cryopreservation of ova, sperm and/or embryos.
Policy:
- Breeding of animals must be scientifically justified, and all breeding activities must be associated with a research project/protocol.
- The production of otherwise available animals specifically for “cost saving” purposes is not permitted since these colonies unnecessarily occupy valuable space that could be used for animals not otherwise available and the actual costs involved include many significant overhead expenses that are subsidized by the University, and not recovered in per diem structures.
- Permission to establish a breeding colony is granted on a case-by-case basis, with the most acceptable reasons for requiring a breeding colony listed below:
- Experiments involving prenatal or early neonatal studies for which subjects would be too young to be procured commercially.
- Breeding/backcrossing of genetically modified lines not available commercially.
- Creation of new transgenic, knock-out or other genetically modified animals.
- Breeding rare inbred lines not available commercially.
- Production of fertilized gametes/embryos for molecular studies
Last Reviewed by the IACUC 7/9/2025
Enrichment - Canine Enrichment and Exercise Program
Canine Enrichment Forms
- Each dog will have their own “Canine Enrichment Form” documenting their participation in the canine enrichment program
- All forms (active and non-active) with arrival dates within the same calendar year will be kept in the Canine Enrichment Form Binder (located in the building receiving area).
- Older forms will be filed for three years after euthanasia
Floor space provisions
- Group -housed dogs - Are to be housed in a pen that provides, at a minimum, 100% of the floor space required by the Animal Welfare Regulations (AWR)/The Guide for the Care and Use of Laboratory Animals (Guide) (whichever provides more space) for each dog. In this case additional opportunity for exercise is not required.
- Individually housed dogs - Are to be housed in a pen that provides, at a minimum, two times the floor space required by the AWR/Guide (whichever provides more space) per dog. In this case additional opportunity for exercise is not required.
- Dogs that are housed individually in enclosures that provide less than two times the required floor space per dog will be provided with additional opportunities for exercise.
- Removal from cage for a minimum of 30 minutes and allowed to roam the room perimeter at will.
- Opportunity for additional exercise will be provided daily during normal working hours.
- One or more persons will observe the animals during the opportunity for exercise.
- A sign will be placed on the room door to note that dogs are loose within the room
- One or more persons will positively interact with the dogs during the opportunity for exercise.
- When possible, several compatible dogs of the same sex from the same room will be released together to facilitate social interaction.
- Removal from cage for a minimum of 30 minutes and allowed to roam the room perimeter at will.
Space requirements
(Please reference the AWR and the Guide for more details)
| AWR (2005) | The Guide (2010) |
|---|---|
|
(dog length [inches] + 6)2 / 144 = ft2 dog length = tip of the nose to the base of tail (inches) |
<15 kg needs 8.0 ft2/ animal Up to 30kg needs 12 ft2 / animal > 30 kg needs > 24 ft2 / animal |
Group housing
- All dogs will be paired or grouped housed when research design and temperament allow
Toys/manipulanda, treats and beds/bedding
- All dogs will be provided with at least 2 toys per dog
- Toys will be rotated on a weekly basis
- Each dog will receive treats (example: canned food, commercial treats) at least once a week
- Amount of treats to be fed:
- Canned food:
- Dog < 20 lbs. – consult with a veterinarian
- Dog 20 to 50 lbs. – ¼ can
- Dog 50 to 100 lbs. – ½ can
- Commercial treats:
- Large treats (anything 2 inches or more in length or diameter), give:
- Dog < 20 lbs. – consult with a veterinarian
- Dog 20 to 50 lbs. – ½ treat
- Dog 50 to 100 lbs. – 1 treat
- Small treats (anything less than two inches in length or diameter), give:
- Dog < 20 lbs. – consult with a veterinarian
- Dog 20 to 50 lbs. – 1 treat
- Dog 50 to 100 lbs. – 2 treats
- Large treats (anything 2 inches or more in length or diameter), give:
- Canned food:
- Amount of treats to be fed:
- Each dog will be provided with access to a bed or bedding as long as temperament allows
Dog aggressive animals
- Dog aggressive animals will be individually housed to prevent injury to other animals.
- Approved exemptions are entered on the Exemption Form and a yellow informational card is placed on the cage.
- Other provisions of this Program will be followed.
- When animals are individually housed, auditory, sensory and/or visual contact will be maintained.
Individually housed dogs with no sensory contact with other dogs
- Whenever possible, dogs will be housed with other dogs in the same room.
- If only one animal remains in the facility, the individually housed dog that does not have sensory contact with another dog must be provided with positive contact with humans at least once daily.
- Play, pet, groom &/or talk to this animal for no less than 15 minutes per day for as long as it is being housed alone in a room.
- Documentation of this positive physical contact will be maintained on the “Record for Animals Housed Alone”.
Exemptions from the Canine Enrichment and Exercise Program
- Exemptions may be granted by:
- The Attending Veterinarian, or his/her designee, on a case by case basis
- Records for exemptions must be maintained and reviewed at least every 30 days by the attending veterinarian, or his/her designee, unless the exemption is permanent.
- The IACUC for approved scientific reasons
- The Attending Veterinarian, or his/her designee, on a case by case basis
Approved exemptions are entered on the Exemption Form and a yellow informational card is placed on the cage.
Possible reasons for exemption include, but are not limited to:
- Post-operative animals during recovery
- Medical condition or injury
- Dogs exhibiting aggressive behavior towards other dogs
- Scientifically justified and approved by the IACUC
Temporary exemptions from the Canine Enrichment and Exercise Program
| Date | Canine ID | Exemption | Reason for Exemption |
|---|---|---|---|
Permanent exemptions from the Canine Enrichment and Exercise Program
| Date | Canine ID | Exemption | Reason for Exemption |
|---|---|---|---|
Last reviewed by OAR and the IACUC 6/14/2023
Enrichment - Environmental Enrichment Program
Purpose: “The primary aim of environmental enrichment is to enhance animal well-being by providing animals with sensory and motor stimulation, through structures and resources that facilitate the expression of species-typical behaviors and promote psychological well-being through physical exercise, manipulative activities, and cognitive challenges according to species-specific characteristics.” (The Guide for the Care and Use of Laboratory Animals, 8th ed.) These guidelines will describe the University of Iowa’s Office of Animal Resource’s standard enrichment practices for each species listed.
In addition to the enrichment program details here, please see details of social housing located here [13].
Rabbits
Each rabbit is offered:
- Vegetable (e.g. carrot, celery, etc.) weekly
- Bio-Serv Bunny Block on a chain

- One toy (e.g. Dumbbell, Jingle Ball or SS Rattle) at all times



- Each rabbit is offered 2-3 small Shredded Wheat squares at each feeding
- Other food enrichment items may be offered such as timothy cubes or hay
Frogs
- 1 Bio-Serv Rodent Retreat per cage

Ferrets
- 1 Bio-Serv Ferret Ball per cage

- For breeding animals, the Ferret Ball will be replaced with a nest box and bedding
- 1 Bio-Serv Beefy Block on a Universal Hanging Chain per cage

Pigs
- One enrichment toy to be offered per cage at all times
- Examples: Bio-Serv Big Red Apple, 12” Best Ball or Jingle Ball



- Milk jugs with food treats inside but must be removed from enclosure by end of day
Mice, Rats
- Each mouse or rat is housed with enriched paper bedding
- If any mouse or rat is not housed with enriched paper bedding, alternative enrichment is provided (e.g. Nyla-bone, hut)

Guinea Pigs, Hamsters
Each guinea pig or hamster is housed with a retreat (e.g. Rodent Retreat Tunnel or Hut)


Sheep and Goats
- Sheep and goats are offered hay several times a week
- A mirror may be used if animals are singly housed
Fish
- Fish are offered brine shrimp daily
Pigeons
- Pigeons are offered grit in addition to their regular diet
- Additional enrichment is not routinely offered as it interferes with ongoing research
Last reviewed by the IACUC 11/8/2023
Euthanasia (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to describe acceptable methods for the euthanasia of animals used in teaching, research and testing at the University of Iowa. All animal euthanasia must be performed by appropriately trained personnel approved on the Animal Protocol.
Performance of Euthanasia
All animal euthanasia must be performed by appropriately trained personnel approved on the Animal Protocol. All euthanasia procedures must be continuously monitored by the person(s) performing the procedure, until confirmation of euthanasia is complete.
Confirmation of Euthanasia
Any animal euthanized on a University of Iowa Animal Protocol requires a method of confirmation of death [14]. Some acceptable methods of confirmation are described below.
Common Acceptable Methods of Euthanasia
Listed below are some commonly used and accepted methods of euthanasia for different species. This list is not inclusive. Please see the “AVMA [15] Guidelines on Euthanasia [15]” for further information.
Rodents weighing > 500grams
- Acceptable Methods of Euthanasia
- Overdose of chemical anesthetics ( 2-3 times the anesthetic dose)
- Overdose of isoflurane (see “Isoflurane Euthanasia” below)
- CO2 exposure (see the "Rodent CO2 Euthanasia" below)
- Barbiturate overdose
- Acceptable methods of Confirmation of Euthanasia include
- Bilateral thoracotomy
- Decapitation
- Vital tissue harvest (inclusive of heart and/or lungs and/or brain)
Rodents weighing <500 grams
- Adults and neonates > 10 days of age
- Acceptable Methods of Euthanasia
- Overdose of chemical anesthetics ( 2-3 times the anesthetic dose)
- Overdose of isoflurane (see “Isoflurane Euthanasia” below)
- CO2 exposure (please see the "Rodent CO2 Euthanasia" below)
- Barbiturate overdose
- Focused microwave irradiation
- Acceptable methods of Confirmation of Euthanasia include
- Cervical dislocation (not acceptable for rats > 200 grams of body weight or hamsters due to their heavy cervical musculature)
- Decapitation
- Bilateral thoracotomy
- Vital tissue harvest (inclusive of heart and/or lungs and/or brain)
- Continued exposure to CO2 for at least 15 minutes after respiratory arrest
- Acceptable Methods of Euthanasia
- Guinea Pig Neonates: Follow above guidelines for adults and neonates > 10 days
- Mouse, Rat and Hamster Neonates ≤ 10 days of age
- Acceptable Methods of Euthanasia
- Overdose of chemical anesthetics ( 2-3 times the anesthetic dose)
- Decapitation
- NOTE: you do not have to use CO2 first. Per NIH guidelines, decapitation alone for this age group is an acceptable means of euthanasia, no confirmation required
- Acceptable methods of Confirmation of Euthanasia include
- Decapitation
- Acceptable Methods of Euthanasia
- Feti (unborn animals that have not breathed)
- Mouse, Rat and Hamster Feti up to 15 days’ gestation and Guinea Pig Feti up to 34 days’ gestation:
- Acceptable Methods of Euthanasia
- Confirmed euthanasia of mother
- Removal of feti from the anesthetized mother
- No further method of confirmation of euthanasia of the feti required
- Acceptable Methods of Euthanasia
- Mouse, Rat and Hamster Feti 15 days of gestation to birth and Guinea Pig Feti 35 days gestation to birth:
- Acceptable methods of Euthanasia
- Decapitation with scissors
- Confirmed euthanasia of mother (feti not required for study)
- Confirmed euthanasia of mother (feti required for study)
- The uterus with the pups or the pups with the amniotic can be removed after euthanasia of the mother
- If at any point a fetus is allowed to breathe it must be decapitated
- Rapid freezing of feti while anesthetized (liquid nitrogen immersion)
- Anesthesia may be effectively induced by hypothermia of the fetus, which can be achieved by submerging the fetus (with the amniotic sac intact) in cold (4-8⁰C/35-39⁰F) physiological saline until the fetus becomes completely immobile
- If at any point the fetus is allowed to breath it must be decapitated
- Methods of confirmation of euthanasia
- No further method of confirmation of euthanasia of the feti required
- If the mother is euthanized, her death must be confirmed
- Acceptable methods of Euthanasia
- Mouse, Rat and Hamster Feti up to 15 days’ gestation and Guinea Pig Feti up to 34 days’ gestation:
Rodent CO2 Euthanasia
- Animals must not be combined from different cages
- If euthanizing an entire cage the animals must remain in their original housing. If euthanizing part of the cage, move to a clean cage with a filter top.
- The maximum number of mice per cage is 5 mice
- Exception: breeder pair with their unweaned litter
- Instructions for Rodent CO2 Euthanasia
- Adjust the CO2 flow rateas follows:
- Rat breeder cage: 8 L/min
- Rat regular cage: 5 L/min
- Mouse cage: 3 L/min
- Continue CO2 until one minute after breathing stops
- Confirm Euthanasia as described previously in this document
- Adjust the CO2 flow rateas follows:
Isoflurane Euthanasia
- Adjust the isofurane flow rate or concentration to 5% or greater
- Continue isoflurane exposure until one minute after breathing stops
Confirm Euthanasia as described previously in this document
Xenopus
- Acceptable Methods of Euthanasia
- Tricaine Methane Sulfonate(TMS, MS 222) 5-10 grams/L buffered with sodium bicarbonate for a pH between 7-7.5
- Barbiturate overdose
- Decapitation or cervical sectioning while anesthetized
- Euthanasia must be confirmed by double pithing
- Acceptable methods of Confirmation of Euthanasia include
- Bilateral thoracotomy
- Double pithing
- Sternotomy
- Vital tissue harvest (inclusive of heart and/or lungs and/or brain)
- Decapitation or cervical section
Zebrafish (and other fish species as deemed appropriate by veterinary staff)
- Zebrafish 8 days post fertilization (dpf) and older
- Acceptable Methods of Euthanasia
- Tricaine Methane Sulfonate (TMS, MS 222) ≥ 250mg/L buffered with sodium bicarbonate for a pH between 7-7.5
- Barbiturate overdose
- Rapid chilling: Submerge fish in 2-4ºC chilled water
- Fish should not be in direct contact with ice
- Fish must remain in the chilled water for 10 minutes following cessation of opercular movement
- Immersion in CO2 saturated water (NOTE: some fish may exhibit hyperactivity with this method)
- Acceptable methods of Confirmation of Euthanasia include
- Observation of no opercular movements for at least 10 minutes following anesthetic overdose or chilling
- Decapitation
- Acceptable Methods of Euthanasia
- Zebrafish 4-7 dpf
- Acceptable Methods of Euthanasia
- Tricaine Methane Sulfonate (TMS, MS 222) ≥ 250mg/L buffered with sodium bicarbonate for a pH between 7-7.5
- Rapid chilling: Submerge fish in 2-4ºC chilled water
- Fish should not be in direct contact with ice
- Fish must remain in the chilled water for 20 minutes following cessation of opercular movement
- Immersion in CO2 saturated water (NOTE: some fish may exhibit hyperactivity with this method)
- Exposure to a dilute (1-10%) sodium hypochlorite or calcium hypochlorite solution
- Acceptable Methods of Confirmation of Euthanasia include
- Observation of no opercular movements for at least 10 minutes following anesthetic overdose or chilling
- Decapitation
- Acceptable Methods of Euthanasia
- Zebrafish fry 0-3dpf
- Acceptable Methods of Euthanasia
- Exposure to a dilute (1-10%) sodium hypochlorite or calcium hypochlorite solution
- Two step euthanasia:
- 1. MS222 or rapid chilling as described above
- 2. Exposure to a dilute sodium hypochlorite or calcium hypochlorite solution
- Acceptable methods of Confirmation of Euthanasia include
- The exposure to a dilute sodium hypochlorite or calcium hypochlorite solution confirms euthanasia
- Acceptable Methods of Euthanasia
Birds
- Acceptable Methods of Euthanasia (AVMA guidelines [15])
- Overdose of isoflurane (see “Isoflurane Euthanasia” above)
- Barbiturate overdose
- CO2 exposure
- Note: Neonatal and diving birds are tolerant of high concentrations of CO2; therefore, prolonged exposure to high concentrations of CO2 will be required to produce death (e.g., in excess of 5 minutes in 60–70% CO2 for 1-day old chicks).
- Acceptable methods of Confirmation of Euthanasia include
- Bilateral thoracotomy
- Vital tissue harvest (inclusive of heart, lungs, and/or brain)
- Decapitation
- Cervical dislocation (AVMA acceptable for bird as euthanasia, moderately rapid)
Non-Rodent Mammals
- Acceptable Methods of Euthanasia (AVMA guidelines [15])
- Overdose of chemical anesthetics ( 2-3 times the anesthetic dose)
- Overdose of isoflurane (see “Isoflurane Euthanasia” above)
- Barbiturate overdose
- Acceptable methods of Confirmation of Euthanasia include
- Bilateral thoracotomy
- Sternotomy
- Vital tissue harvest (inclusive of heart, lungs, and/or brain)
Euthanasia by Perfusion
- Rodents
- Any animal undergoing perfusion must be under a surgical plane of anesthesia before any incisions are made. A surgical plane of anesthesia must be maintained until the heart stops.
- Any person performing euthanasia by perfusion must be properly trained
- Non-Rodents
- Any animal undergoing perfusion must be under a surgical plane of anesthesia before any incisions are made. A surgical plane of anesthesia must be maintained until the heart stops.
- An OAR veterinarian must observe one euthanasia by perfusion performed by the person responsible for training lab personnel
- Any person performing euthanasia by perfusion must be properly trained
Physical Methods of Euthanasia On Un-Anthesthetized Animals > 10 days old
- Physical methods of euthanasia on an un-anesthetized animal in an Animal Protocol must be justified and approved by the IACUC
- The IACUC Compliance and Training Coordinator must observe one euthanasia without anesthesia performed by the person responsible for training lab personnel
- Any person performing a physical method of euthanasia (i.e. decapitation, cervical dislocation) on an un-anesthetized animal must be properly trained
References
"AVMA Guidelines for the Euthanasia of Animals: 2020 Edition" AVMA.org., 2020.
Last Reviewed by the IACUC 6/11/2025
Euthanasia - Confirmation of Death (Policy)
IACUC Policy:
Confirmation of Death [16]
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: This policy lists the IACUC’s requirements of confirmation of death. The purpose of confirming death is to ensure that the animal cannot recover or reach consciousness.
The policy requires that any animal euthanized at the University of Iowa must be subject to a confirmatory method of death immediately after the method of euthanasia and preceding any other procedure. Any animal found dead must also go through the confirmation of death procedure unless there is secondary physical indication of death (i.e. rigor, autolysis, or desiccation) in addition to cardiac and/or respiratory arrest.
USDA-regulated Species (e.g ferrets, Guinea pigs, swine, hamsters, etc.)
- A physical confirmation of death is required immediately following a method of euthanasia and preceding all other proceduresConfirmatory methods include, but are not limited to:
- Bilateral thoracotomy
- Sternotomy
- Vital tissue harvest (inclusive of heart, lungs, and/or brain)
- Decapitation
- Exsanguination
- Perfusion
Mice and Rats
- Confirmation of death is required immediately following a method of chemical (e.g. gas or injection) euthanasia
- Confirmatory methods for animals older than 10 days of age include, but are not limited to:
- Cervical dislocation
- Not acceptable for rats > 200 grams of body weight
- Decapitation
- Thoracotomy
- Vital tissue harvest (inclusive of heart, lungs, and/or brain)
- Continued exposure to CO2 for at least 15 minutes after respiratory arrest in the original container
- Cervical dislocation
- Confirmatory methods for neonates through 10 days of age or younger include, but are not limited to:
- Decapitation
- Preferred confirmatory method
- Thoracotomy
- Vital tissue harvest (inclusive of heart, lungs, and/or brain)
- Continued exposure to CO2
- This method is discouraged as neonates are more resistant to CO2 hypoxia because it requires extended exposure up to 90 minutes.
- Decapitation
- For isoflurane euthanasia:
- If using a vaporizer: a physical method or continued exposure to isoflurane for at least 15 minutes after respiratory arrest is acceptable
- If using a drop jar: a physical method (cervical dislocation, decapitation, thoracotomy or vital tissue harvest) must be used for confirmation
Xenopus
- A physical confirmation of death is required following a method of euthanasia
- Confirmatory methods of euthanasia following an overdose of anesthesia:
- Double pithing
- Removal of heart
Zebrafish
- Confirmation of death is required following a primary method of euthanasia
- Confirmatory methods for Zebrafish 8 days post fertilization (dpf) and older include, but are not limited to:
-
- Observation of no opercular movements for at least 10 minutes following anesthetic overdose or chilling
- Decapitation
-
- Confirmatory methods for Zebrafish 4-7 dpf include, but are not limited to:
-
- Observation of no opercular movements for at least 10 minutes following anesthetic overdose or chilling
- Decapitation
- Confirmatory methods for Zebrafish 0-3 dpf include, but are not limited to:
- Exposure to a dilute (1-10%) sodium hypochlorite or calcium hypochlorite solution
-
References
- " AVMA Guidelines for the Euthanasia of Animals: 2020 Edition" AVMA.org., 2020.
Last Reviewed by the IACUC 6/11/2025
Euthanasia by OAR Personnel (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: The purpose of this document is to describe euthanasia of animals performed as a service by the Office of Animal Resources at the University of Iowa.
The Office of Animal resources provides a euthanasia service for cull animals (animals for which no tissues or postmortem analysis is needed). It is the responsibility of the investigator to euthanize animals at experimental endpoints or when directed to by the veterinary staff based on a health concern. Euthanasia of cull animals will be performed in a humane manner as determined by the veterinary staff and in accordance with the IACUC Policy: Confirmation of Euthanasia.
Last reviewed by the IACUC: 2/14/2024
Genotyping - Mouse Toe Clipping (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose:
The intent of this IACUC policy is to describe when mouse toe clipping may be performed and the standard procedures for performing this technique. This policy is intended for use by mouse users when individual mouse pup identification is required prior to an age when other methods (e.g. ear punch, tag, microchip) are not appropriate.
Policy:
Toe clipping for the purposes of identification and/or genotyping may be approved in the animal protocol under the following circumstances:
- Mice up to 7 days of age may be toe clipped for identification purposes
- Mice 8-14 day of age may be toe clipped ONLY if the toe tissue is also used for genetic analysis
- This is considered a refinement by making it unnecessary to perform tail biopsies for tissue sampling
The following procedures MUST be followed:
- No more than 1 toe per paw may be clipped
- Avoid clipping digits/toes on fore paws if possible
- DO NOT clip the 1st digit/toe (i.e. thumb) on either fore paw
- Only remove the 3rd phalanx (i.e. last bone of a digit); in other words, amputate at the joint between the 2nd and 3rd bones/phalanges
- Aseptically prepare the digit before clipping (i.e. wipe with betadine or alcohol)
- Use very sharp scissors (fine pointed tips work best)
- Scissors must be cleaned, preferably sterilized (i.e. hot bead sterilizer), between animals
- Alternately, scissors may be sanitized with 70% ethanol or antiseptic solution (e.g. povidone iodine, chlorhexidine)
- Monitor animals continuously until bleeding has stopped
- Bleeding may be stopped using a piece of gauze with gentle pressure between finger tips
- OAR veterinary staff must be contacted promptly if toe does not heal properly or if the animal cannot ambulate normally following the procedure
Any procedure involving toe sampling that does not meet the above criteria must be described and appropriately justified in an IACUC-approved Animal Protocol.
References:
- National Institutes of Health, ARAC, Guidelines for Toe Clipping of Rodents, Revised 6/13/07 (http://oacu.od.nih.gov/ARAC/index.htm [17])
- Guide for the Care and Use of Laboratory Animals, 8th ed, National Research Council, National Academy Press, 2011, page 75.
- Federation of European Laboratory Animal Science Associations Working Group, FELASA Guidelines for the Refinement of Methods for Genotyping Genetically-modified Rodents 2013. Laboratory Animals 47(3) 134-145 .
Last Reviewed by Committee: 3/13/2024
Genotyping - Rodent Tail Snipping for Genotyping (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: The intent of this policy is to describe procedures required for tail tissue collection in rodents for genetic analysis. This policy is intended for use by research staff and Office of Animal Resources staff approved to perform this procedure on an Animal Protocol. This policy is approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC).
Procedures:
- Mice and Rats 10-24 days old:
- anesthesia or analgesia recommended but not required
- Note: Better quality DNA and higher DNA yield has been reported from tail snips at 18 days of age or younger, due to a lower percentage of ossified sample. In addition to better quality yields, typically this age range results in less bleeding issues.
- If delayed weaning has been approved by veterinary staff or as a part of the approved Animal Protocol, you must still perform tail snipping by 24 days of age. Justification to do otherwise is provided in detail below.
- Tail snipping procedure:
- Gently, but securely, restrain animal (manual or mechanical)
- Snip tail with sanitized sharp scissors or disposable blade
- Minimize the amount of tissue removed - 2 mm of distal tail has been identified as sufficient tissue to perform multiple PCR reactions 2
- DO NOT remove more than 5mm of tail
- Place tail tip into a tissue collection tube
- Ensure hemostatis (stop bleeding):
- Apply pressure to the cut portion of the tail with gauze until bleeding has stopped
- If continuous pressure does not stop the bleeding, utilize a chemical cautery agent (e.g. silver nitrate or Kwik Stop®)
- Heat cautery can also be used
- Return animal to its cage and monitor for bleeding for at least 5 minutes
- Clean off biologic material (e.g. blood or fur) from scissors and sanitize after each snipping
Note: Any procedure(s), other than a one-time collection in animals between 10-24 days of age, must be described and justified in the Animal Protocol. The following are examples of acceptable deviations if appropriate justification is provided in the Animal Protocol:
- Sampling of neonatal mice under 10 days of age (consult with OAR veterinarian)
- Sampling of animals greater than 24 days of age
- Use of a systemic analgesic given prior to tail snipping is required
- Tailing at this age is potentially painful and should be avoided if possible
- More than one sample over the life of the animal
- Limit of two collections, no more than a total of 2-5mm of the distal tail removed over all collections
- Use of a systemic analgesic given prior to repeat tail snipping is required
References:
- Bonaparte, Dolores, Paolo Cinelli, Eleni Douni, Yanne Herault, Alex Maas, Pirjo Pakarinen, Matti Poutanen, Mirentxu S. Lafuente, and Ferdinando Scavizzi. “FELASA Guidelines for the Refinement of Methods for Genotyping Genetically- modified Rodents: A Report of the Federation of European Laboratory Animal Science Associations Working Group." FELASA Guidelines for the Refinement of Methods for Genotyping Genetically-modified Rodents: A Report of the Federation of European Laboratory Animal Science Associations Working Group 47.3(2013): 134-45.
- Hankenson, F CLaire, Laura M. Garzel, David D. Fischer, Bonnie Nolan, and Kurt D. Hankenson. "Evaluation of Tail Biopsy Collection in Laboratory Mice (Mus Musculus): Vertebral Ossification, DNA Quantity, and Acute Behavioral Responses." Journal of the America Association for Laboratory Animal Science 47.6(2008): 10-18.
- Jones, Carissa P., Scott Carver, and Lon V. Kendall. "Evaluation of Common Anesthetic and Analgesic Techniques for Tail Biopsy in Mice." Journal of the America Association for Laboratory Animal Science 51.6 (2012): 808-14.
Last Reviewed by the IACUC 12/11/2024
Hazardous Agent Containment (biohazards, chemical hazards, & radioactive materials)
Multiple hazard containment protocol forms have been developed by the Office of Animal Resources (OAR) and Environmental Health & Safety (EHS) for use of hazardous materials in animals. These hazard containment protocol forms should be submitted along with the Animal Protocol for review by the Institutional Animal Care and Use Committee (IACUC). A copy of the containment forms is available from the eIACUC Animal Protocol system (Special Circumstances & Hazards section) or on the IACUC website - Hazard Containment Protocol. [18]
For additional information on animal housing containment guidelines, please visit the EHS website - Animal Housing Containment Guidelines [19].
If you have further questions on hazardous agents and the requirements of their use, please contact the appropriate EHS staff. Contact information and areas of expertise can be found on the EHS Contact Us [20] page.
Humane Intervention Points (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose
The purpose of these guidelines is to provide a set of regularly used criteria for the establishment of humane intervention points for research animals at the University of Iowa. These guidelines are intended for use in writing humane study endpoints, whose purpose is to prevent or minimize animal pain or distress during research activities.
Definitions
- Humane Intervention Points1: a set of predetermined physiological or behavioral criteria that define the point at which humane intervention must be implemented to prevent or relieve unnecessary pain or distress in a research animal. Humane intervention points function as an effective way to refine research and ensure accurate and timely data collection.
- Humane Interventions include but are not limited to those listed below:
- Provide adequate veterinary treatment to alleviate pain or distress, such as analgesia and/or supportive therapy to the animals (ie. fluids, dietary supplementation such as providing Diet Gel, heat, etc.)
- Cease performing experimental procedures that contribute to pain or distress until the animal recovers
- Remove the animal from the study
- Increase the frequency of observation to ensure early identification of pain or distress
- Euthanize the animal
- Experimental endpoint: the point at which scientific aims and objectives have been reached
- Not all animals will reach this point if humane intervention points appear/are identified and treatment does not alleviate pain or distress
- Pilot Study: a preliminary study used to determine appropriate humane intervention points in cases where the course of disease, experimental effects, or indicators of distress are unknown
- An IACUC approved Animal Protocol is required to perform a pilot study
- Training Animals: animals used in cases where individuals need to develop skills and become competent in procedures, in addition to those used to refine a procedure. Includes an animal carcass or an animal used in a terminal/non-survival procedure.
- An IACUC approved Animal Protocol is required to use training animals
- Training animals reach their experimental endpoint when the training objective is complete; they are not used to collect subsequent data
- Different humane intervention points may be needed for training than for data collection
- Scoring System: a method of animal evaluation in which numerical values are assigned to specific clinical signs and/or behavioral observations
- Humane intervention points are predetermined and based on the total numerical score assigned to each established criteria
- Numerous scoring systems are already established and published in scientific literature
- Consult OAR and/or IACUC Veterinarians for guidance in establishing a new scoring system
Establishing Humane Intervention Points
- Determine what humane intervention points and what interventions are appropriate for the study
- Use objective, measurable parameters when possible to designate the points at which intervention occurs
- Ensure monitoring frequency, humane intervention points, and subsequent/following actions are clearly defined in the Animal Protocol, particularly under applicable experimental procedures
- Ensure all personnel responsible for making animal observations have been adequately trained to observe, recognize, and document the humane intervention point parameters as described and approved in the Animal Protocol
Commonly used Humane Intervention Points
Criteria at which intervention or euthanasia must occur unless scientific justification is provided and approved in the Animal Protocol include (but are not limited to) the following:
- Weight loss
- For acute infection studies, ≥20% weight loss compared to the weight at the start of the experiment
- For chronic/long-term studies (especially of juveniles/growing animals), ≥20% weight loss compared to control animals or animals of a similar age (preferably sharing the same background strain)
- Body Condition Scoring (BCS)
- For mice, a BCS of less than or equal to “2” out of “5” (BCS ≤ 2) on the scale from Ullman-Cullere, et al. 3
- For rats, a BCS of less than or equal to “2” out of “5” (BCS ≤ 2) on the scale from Hickman and Swan, et al. 4
- For other animals, the BCS scale used should be defined or referenced
- Inability to rise or move about the cage/pen
- Access to food and water is impaired
- Animal unable to right itself
- Discrete Tumors
- Ulceration, necrosis, or infection
- Interference with normal posturing or ambulation
- Size
- For mice, 2 cm in diameter by any measurement/in any dimension
- For rats, 3 cm diameter by any measurement/in any dimension
- Metastatic/Disseminated Tumors
- BCS ≤ 2
- Increased respiratory rate or effort
- Fluid in the abdomen (ascites)
- Dehydration that recurs frequently or is not responsive to therapy
- Delayed skin tent, sunken eyes, tacky mucous membranes
- Labored breathing
- Neurologic signs severe enough that they prevent normal eating/drinking
- Bleeding that cannot be readily stopped or recurs/returns frequently
- Self-induced trauma that creates wounds
- Post-surgical complications
- Dehiscence or infection of surgical site
- Signs of systemic infection (lethargy, hunched posture, increased respiratory rate)
Moribund
Stating that animals will be euthanized when they become moribund is not an appropriate humane intervention point as this term is subject to individual interpretations. In addition, it can be assumed that the moribund animal has experienced significant distress in the period leading up to the moribund condition. The purpose of identifying humane intervention points is to prevent or minimize animal pain or distress.
Death as Endpoint
The continuation of a study until an animal dies is almost never acceptable. Strong scientific justification is required for such a study. Contact the Office of the IACUC for further guidance if animal death is a necessary endpoint.
References:
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition (p. 27). Washington, DC: The National Academies Press; 2011.
- Williams WO, Baneux P. Humane Intervention Points: Refining endpoint terminology to incorporate non-euthanasia intervention options to improve animal welfare and preserve experimental outcomes. Lab Anim. 2022 Oct;56(5):482-489
- Ullman-Cullere MH, Foltz CJ. Body Condition Scoring: a rapid and accurate method for assessing health status in mice. Lab Animal Science; 49(3): 319-323, 1999.
- Hickman DL, Swan M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci. 2010; 49(2):155-9.
Last Reviewed by the IACUC 2/12/2025
Media - Media Security (Policy)
Policy: The Institutional Animal Care and Use Committee (IACUC) has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose:
This Media Security Policy (MSP) has been established by The University IACUC and is intended to protect the confidentiality and integrity of University of Iowa research, to assure respect for the privacy and safety interests of faculty, staff and students (University personnel) and to prevent misleading representation of animal care and use at the University and its affiliates.
This Policy describes the allowable use of recording devices in any area where animals are housed, tested or used at the University of Iowa (including laboratories, University vehicles/conveyances and field study locations) and provides University personnel guidance on how to best present and share recordings involving live or dead animals or their parts.
Please also see the IACUC Policy regarding the use of Social Media [21]
Users should be aware that media created for research purposes may be subject to Public Records Requests.
Policy:
The use of any recording device (e.g., film camera, digital camera, camera phones, digital recorder, sound recorder, live streaming equipment) to record sounds or images of animals or animal use areas is prohibited unless one of the following exceptions apply:
- When performed by the Principal Investigator (PI) or designee when required for University-sanctioned scientific reasons (e.g., publications, laboratory documentation)
- When performed by the PI or designee for the purpose of recording instructional activities as described in the respective IACUC-approved protocol
- When performed by or at the direction of the Office of Animal Resources (OAR) or Office of the IACUC veterinary staff for the purpose of diagnosing or documenting clinical disease, veterinary care or treatment
- When performed by personnel in consultation with the OAR when required to document condition of facilities, or compliance or animal welfare issues
- When performed by IACUC members or office staff to document conditions during IACUC mandated inspections
- When performed by government inspectors (e.g. USDA Veterinary Medical Officer)
University personnel who wish to record or allow recording by others (e.g. journalists) in animal use areas for reasons not described in the above list of exceptions must involve the Director of the IACUC Office or Attending Veterinarian prior to recording. University personnel are also strongly encouraged to work with designated press officers throughout the recording session or subsequent information dissemination process by contacting the University’s Strategic Communications Director.
Guidance for implementing this policy
University personnel wishing to take or allow photographs or recordings of animals should consider the following:
- Whether the desired photography or recording meets one of the exceptions listed above.
- Recordings should be promptly downloaded to a secured drive and not stored for longer than necessary on the camera, video recording device, an unsecured computer hard drive or other unsecured external storage device.
- All photography and recording should be deleted as soon as no longer needed.
- When recording live or euthanized animals, record only as much of an image as is needed for the applicable exception listed above.
- Appropriate safety, handling, and restraint methods for the species should be used.
- No unintentional references to the University of Iowa should be visible in the photograph. Pay attention to background and items.
Rights of Individuals
Persons who could be photographed or recorded in the course of their work or otherwise under this Policy should be informed when such activity is imminent. Any individual may decline being photographed, filmed or recorded and is not required to be subject to photography or recording.
- Although recording of animals or animal activities may be permitted by this Policy, University personnel should recognize that what they perceive as appropriate may not necessarily be seen that way in the public eye. Special attention should be given to the secure processing, transport and storage of negatives, disks, tapes, media cards, etc. and dissemination of images or recordings taken by investigators or authorized personnel in the course of their work.
- Every effort should be made to show appropriate and accurate context when audio or visual recordings are made (e.g., if an animal is anesthetized or sedated, include the anesthetic vaporizer or tray holding the bottle of the tranquilizing agent; have personnel wearing required personal protective equipment appropriate for the work appear in the image).
- For security purposes, every effort should be made to avoid showing identifying landmarks (e.g., building and room numbers, worker’s name badges, etc.).
- University personnel should be mindful of the potential for photographs and recordings of animals to be the subject of public records requests. Such photographs or recordings may be accessible to members of the public under either Iowa’s Public Records Law or the Federal Freedom of Information Act (FOIA). University personnel may further consult with the University’s General Counsel about applicable laws.
- Careless or casual use of recordings from animal use areas could unintentionally expose the University or its employees, students, vendors, etc. to unwanted attention and harassment or could misrepresent the nature of such activities occurring at or by the University of Iowa. University personnel may consider asking students and visitors to read this Policy prior to entering animal use areas, engaging in animal-related activities or taking photographs or other recordings. University personnel may also consider adding this policy to departmental or course-specific policies or agreements.
Last Reviewed by the IACUC 3/8/2023
Media - Social Media (Policy)
Policy: The Institutional Animal Care and Use Committee (IACUC) has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose:
In an effort to provide education about the benefits and risks of social media and to minimize personal and future career risk, suggested guidelines for use of social media can be found at the University of Iowa (“University”) Human Resources Website. These guidelines are intended to support creative and innovative use of social media by staff and to further University purposes in a manner that minimizes personal, professional, and institutional risk. Whether social media is being used for business or personal use, being thoughtful about what and how much to share on a social media platform is important. In some instances, information taken out of context can be damaging to the individual’s reputation and/or the University’s ability to pursue its mission. It is important to remember that posts can appear to be removed or deleted, but in fact could remain available and circulating due to high probability that the site or its contents have been saved or archived, or someone else has saved, downloaded, or shared the information.
Faculty, staff, students and visitors at the University of Iowa shall not post to personal social media accounts any written media, sounds, images, or other information that would violate the IACUC Media Security Policy [22] or are otherwise not approved for personal use (e.g., posters, presentations, or other materials intended for scientific use only). In an effort to protect the University of Iowa, its employees, and its students, failure to adhere to this policy, whether on-duty or off-duty, could result in loss of animal use privileges.
Last Reviewed by the IACUC 3/8/2023
New Weanling Procedure for Labs (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: This document provides guidance to aid laboratory staff in required and recommended practices for the care of new weanling mice. As a reminder, proper rodent breeding management requires not only breeder pair evaluation, dating of litter births and proper weaning practices, but includes care in the post-weaning period, a time of high stress for young animals. This includes involvement up to 5-6 weeks of age for most mouse strains, sometimes longer for genetically modified lines. Failure to perform proper breeding management steps may result in OAR involvement and fees for your lab.
***This guidance document can be found here: Office of Animal Resources, Animal Health & Care "Investigator guidance for monitoring and managing new weanling mice [23]" ***
Outbreak - OAR Pathogen Outbreak Control Plan
The following plan represents the IACUC-endorsed steps that will be taken by the Office of Animal Resources when an excluded pathogen (viruses, bacteria, parasites) is detected in our animal colonies
-
Restrict room access to reduce risk of collateral transmission
- OAR will remove electronic access to the affected room doors as soon as the health reports indicate the detection of an excluded pathogen
-
Place quarantine signage and procure supplies
- OAR will label the doors to the affected rooms with signage detailing the agent detected and precautions to enter the room
- OAR will provide necessary supplies to manage the quarantine status
-
Communicate with affected labs on findings
- OAR will communicate with the affected investigators, key lab personnel and department DEOs for research staff affected by the pathogen detection
THE ABOVE ACTIONS (I-III) WILL OCCUR ON THE FIRST DAY THAT AN EXCLUDED PATHOGEN IS DETECTED
-
Animal room access restrictions
- OAR will permit access to the quarantine rooms for up to two lab staff members
- Lab staff assigned to the quarantine rooms will only have access to the quarantine rooms within OAR housing
- Lab staff designated to work in the quarantined rooms will be trained on proper quarantine procedures prior to being granted access to the affected rooms
- OAR will not assign affected investigators additional housing rooms within the vivarium
- OAR will permit access to the quarantine rooms for up to two lab staff members
-
Animal movement restrictions
- Animals will not be allowed to move outside of the quarantined room except under special circumstances as approved by an OAR veterinarian
Examples:
Approved terminal tissue collection
Euthanasia
- OAR will request that any animals from affected rooms that were in the labs or procedural spaces when the room was locked down are returned to the animal rooms immediately
- Lab staff can contact an OAR supervisor or veterinarian for assistance with this task
-
Bio-sampling of affected colonies and treatments
-
Parasites
- Affected sentinels and select colony cages will be sampled to confirm the presence of the excluded pathogen
- Fur mites - ectoparasites
- Fur samples or fur swabs will be collected from animals
- Pinworms - endoparasites
- Fecal samples will be collected
- Fur mites - ectoparasites
- All colony animals housed within the affected rooms will be treated after research notification and approval
- Affected sentinels and select colony cages will be sampled to confirm the presence of the excluded pathogen
-
Viruses/bacteria
- Affected sentinels and at least one animal per cage will be sampled to confirm the presence of an excluded viral agent
- Sampling could involve collecting a drop of blood and/or fecal sample
- Breeding moratorium will be enacted
- Cages that test positive for the excluded virus will be reported to the labs and the animals euthanized to reduce transmission risk
- Affected sentinels and at least one animal per cage will be sampled to confirm the presence of an excluded viral agent
-
-
Designation of procedural space for terminal tissue harvest
- OAR will consider the designation of a quarantine procedural space based on identified pathogen, investigator needs and space availability within the affected vivarium
- Designated quarantine lab staff will be trained on proper quarantine procedural space techniques prior to being granted access to the procedural space
- Tissues harvested must be fixed to prevent propagation of viable pathogens
-
OAR will retest for the presence of pathogens as appropriate to the agent, until clearance can be confirmed and quarantine restrictions lifted
-
Room decontamination
- Once the pathogen has been eliminated, OAR will remove and dispose of materials that cannot be decontaminated
- Animals will be placed in clean cages and moved to a sanitized room, where all lab staff will have unrestricted access to them
- Housing and procedure room(s) and equipment will be decontaminated by OAR prior to further animal use.
Pain Recognition in Laboratory Animals (Informational Sheet)
OAR Informational Sheet: Pain Recognition in Laboratory Animals
Informational Sheet: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. Informational Sheets provide information about frequently asked questions and represents guidance for best practices. Deviation from the recommendation(s) does not require specific justification.
Purpose: The purpose of this document is to help researchers and OAR husbandry staff recognize signs of pain in animals. This document does not list all signs of pain but rather lists more common and easy to identify signs of pain in animals.
All investigators should consider that procedures that cause pain or distress in human beings may cause pain or distress in other animals.1 Procedures expected to cause more than slight or momentary pain (e.g., pain in excess of a needle prick or injection) require the appropriate use of pain-relieving measures unless scientifically justified in an approved animal care and use protocol.2
Relief of Pain
Details of recommended pain relief methods and doses can be found in the IACUC Analgesia Guidelines, including non-pharmacologic (non-drug) methods which should be considered in all cases of potentially painful procedures, including those for which analgesic drugs are contraindicated by study design.
-
Mice
- Hunched posture
- Reduced grooming and ruffled fur
- Reduced level of spontaneous activity
- Reduced food/water intake
- Separation from cage mates
- Squinty-eyes
- Pale eyes (if albino)
- Increased aggressiveness when handled
-
Rats
- Hunched posture
- Reduced grooming and ruffled fur
- Reduced level of spontaneous activity
- Falling/staggering, poor gait and twitching
- Reduced food and water intake
- Red-staining around nose and/or eyes (i.e. porphyrin secretions)
- Squinty-eyes
- Pale eyes (if albino)
- Back arching behavior
- Horizontal stretching behavior
- Abdominal pressing behavior (briefly pressing abdomen to the ground)
- Increased aggressiveness when handled
-
Rabbit
- Decreased fecal production
- Reduced food and/or water intake
- Reduced activity
- Hunched posture
- Tensing of muscles (guarding)
- Bruxism (grinding of teeth)
- Reduced grooming
- Squinty-eyes
- Pale eyes (if albino)
- Aggressiveness
-
Farm animals (i.e., pigs, sheep, etc.)
- Hunched posture
- Separation from flock or herd
- Lack of interest in surroundings
- Decreased mentation (mental activity)
- Decreased appetite
- Bruxism (teeth grinding)
- Drooping ears
- Head drooping below withers
- Vocalization
- Grunting (spontaneously, or when painful region palpated)
- Lameness
- Long durations of lying down and reluctant to stand when prompted
- Restlessness
- Tachycardia (rapid resting heart rate)
-
Dog
- Hunched posture with lowered head
- Decreased or absent appetite
- Decreased grooming
- Licking wound or surgical site
- Lameness
- Guarding (protecting) the painful area
- Stiff gait and slow to rise
- Trembling or shaking
- Limited or no movement when awake
- Weak tail wag or low carriage of tail
- Sitting or lying in an abnormal position
- Praying position (i.e. front legs and head on floor, hindquarters in the air)
- Lack of normal vocalization (no greeting bark or noise)
- Whining, barking or growling
- Dull mentation or agitation
- Inappropriate urination or defecation, or not moving away from it
- Acts out of character (gentle dogs may bite or become aggressive)
-
Cat
- Hunched posture with lowered head
- Decreased or absent appetite (associated with weight loss when chronic)
- Decreased grooming
- Licking wound or surgical site
- Bearing no or partial weight on affected limb or any degree of limp
- Guarding (protecting) the painful area
- Sitting or lying in an abnormal position
- Trembling or shaking
- Limited or no movement when awake
- Stiff gait and slow to rise
- Lack of normal vocalization (no noise of greeting or wanting to be fed)
- Yowling or crying (with acute pain)
- Hissing or growling, especially when painful area is touched
- Hyperventilation or open mouth breathing
- Acts out of character (aggressive or playful cats may become docile or quiet)
- Inappropriate urination or defecation, or not moving away from it
- Dull mentation or agitation
-
Ferrets
- Inappetance
- Lameness
- Reluctance to move
- Trembling
- Vocalization
- Teeth grinding
- Hunched
- Lip licking
- Repeated or excessive yawning
-
Guinea Pigs
- Anorexia or inappetance
- Lameness
- Increased vocalization
- Decreased activity
- Decreased water consumption
- Mutilation of painful area
-
Hamsters
- Weight loss
- Excessive scratching and licking
- More aggressive when handled
- Vocalization when handled
- Mutilation of painful area
-
Birds and Poultry
- Vocalization
- Crouched posture
- Closed or partially closed eyes
- Inappetence
- Inactivity
- Lameness
- Reduced perching
-
Amphibians
- Amphibian species such as frogs, toads, and salamanders are commonly used in laboratory animal research settings, but there is no objective means to assess the presence and severity of pain in amphibians, especially since they do not exhibit any facial expression. However, research studies have shown that amphibians are able and motivated to learn to avoid noxious stimuli.
Some exotic animal clinicians use nonspecific clinical signs such as decrease in avoidance movement (e.g., when approached by a handler) or decrease in appetite as indicators of pain in these animals.4
- Amphibian species such as frogs, toads, and salamanders are commonly used in laboratory animal research settings, but there is no objective means to assess the presence and severity of pain in amphibians, especially since they do not exhibit any facial expression. However, research studies have shown that amphibians are able and motivated to learn to avoid noxious stimuli.
-
Fish
- It is difficult to determine the nature of the response to pain in fish or whether their experience is similar to that observed in mammals. Although there have been few species-specific studies, there is evidence that fish exhibit a pronounced initial response to injuries or to contact with nociceptive stimuli or chemical algesics but their response to chronic stimuli has not been characterized.
Generally, fish react to noxious stimuli (such as puncture with a hypodermic needle) with strong muscular movements, and when exposed to a noxious environment (such as an acidic solution) show abnormal swimming behavior, attempts to jump from the water, and more rapid opercular movements. Such effects indicate some, perhaps considerable, distress, but it is not possible to describe the distress unequivocally as pain-induced.4
- It is difficult to determine the nature of the response to pain in fish or whether their experience is similar to that observed in mammals. Although there have been few species-specific studies, there is evidence that fish exhibit a pronounced initial response to injuries or to contact with nociceptive stimuli or chemical algesics but their response to chronic stimuli has not been characterized.
References
- U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training
- Pain and Distress in Laboratory Animals. ACLAM position statement approved October 29th, 2001.
- Guidelines for the Assessment and Management of Pain in Rodents and Rabbits ACLAM position statement approved July 2006.
- “Recognizing Pain in Animals” ILAR, <http://dels-old.nas.edu/animal_pain/>. [24] 2009.
Last updated 8/14/2024
Principal Investigator Eligibility for Animal Protocols (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose:
Due to the need for accountability and the level of responsibility involved in conducting research, teaching, and/or training with live vertebrates, the Principal Investigator (PI) must be physically present at the University of Iowa (UI) with sufficient frequency and duration* to provide adequate oversight. The IACUC must ensure that the PI is able to actively oversee the personnel and procedures being conducted under their approved Animal Protocol(s). This policy lists types of appointments not ordinarily allowed to serve as Principal Investigator (PI) on a University of Iowa (UI) Animal Protocol.
Policy
UI employees who have left the University (i.e. no longer have an appointment), are on extended leave of absence, Emeritus faculty or faculty with Visiting, Adjunct, Courtesy or other similar appointments will ordinarily not be allowed to serve as a PI on an Animal Protocol.
If a PI’s appointment changes to one of the above mentioned statuses and he/she still has active Animal Protocols, an appropriate person will be contacted by the IACUC office and informed of the following:
- No animals may be ordered or utilized on the AP(s) until an amendment request to change the PI has been approved by the IACUC.
- Any animals currently housed will be “red carded” and assigned to the IACUC Animal Holding Protocol.
- An amendment request to change the PI or a request to close out the AP(s) must be received within 30 days of date of notification of change of status. If no amendment is received, the AP(s) will be closed and any remaining animals will be euthanized or transferred by the IACUC to another approved Animal Protocol with a valid UI PI.
The IACUC Chair will consider requests by individuals not ordinarily allowed to serve as PI on a case by case basis, depending on the proposed animal activities and the rationale for the request.
*Sufficiency of the frequency and duration of PI presence is to be determined in consultation with the IACUC Chair.
Last Reviewed by the IACUC 3/10/2021
Social Housing of Species (Policy)
Housing of Social Species (Policy)
Policy:
The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose
The Guide to the Care and Use of Laboratory Animals states that members of a social species should be socially housed whenever possible. The purpose of this policy is to define what constitutes an acceptable justification for single housing of social species. It is the policy of the IACUC that social experience is the standard for social species. However, the IACUC recognizes that not all members of social species are compatible and that there are circumstances when social housing is not possible.
Potential social species at the University of Iowa: dogs, ferrets, pigs, sheep, goats, rabbits, most rodents (excluding: male mice, female hamsters), chickens, turkeys and pigeons.
The following are acceptable justifications for single housing of social species:
-
Justification in Animal Protocol required:
- Scientific necessity
- Justification for single housing should be defined for the shortest period of time necessary
- Scientific necessity
-
Justification in Animal Protocol NOT required:
- Veterinary concerns regarding the well-being of the animal
- Observed social incompatibility (e.g. fighting, food guarding, etc.)
- Immediate post –operative recovery
- Attrition of cage mates
- Remaining animals may be combined, if appropriate (DO NOT COMBINE MALE MICE PAST WEANING AGE)
- Only one animal of a sex or genotype is produced in a litter and no other appropriate weanlings are available for group housing
General Considerations:
-
When social animals must be housed singly:
- Environmental and/or food enrichment, exercise/release into a larger enclosure, and/or human interaction will be provided, unless scientifically contraindicated
- Con-specifics will be housed in visual, auditory, olfactory and/or tactile range, whenever possible
-
If primary cage size is the limiting factor for the ability to group house, OAR shall develop a plan to address the need for larger caging
-
Animals that have been singly housed may need gradual introduction to cage mates
- Veterinary consultation should be used to determine if compatible cage mates can be found
Species Specific Considerations:
-
DOGS:
- Unfamiliar dogs that will be housed for less than 2 weeks need not be pair housed due to the potential for aggression.
- Single housed dogs will receive enrichment according to the canine enrichment program
- Unfamiliar dogs that will be housed for less than 2 weeks need not be pair housed due to the potential for aggression.
-
FERRETS:
-
Pregnant Jills may be singly housed for up to 14 days prior to whelping.
- Males may be group housed at weaning and stay together until they are separated for breeding or research purposes. Group housing males at ages up to pre-puberty, or regrouping previously separated males up to this age may be attempted with veterianry consultation.
- Attempts to group house sexually mature male ferrets should be done under veterinary supervision
-
-
RABBITS :
- Younger than 4 months of age (sexually immature): Should be group housed by sex if they will be housed in the vivarium for longer than 3 months
- Older than 4 months of age (sexually mature): Unless previously socially housed and shown to be compatible, should be singly housed
- Attempts to group house sexually mature rabbits should be done under veterinary supervision
-
MICE:
- Male mice are often aggressive and are not considered social
- Male mice may be housed together only when co-housing occurs at weaning age or earlier
- Once a male mouse is removed from a male group housing cage (for breeding or prolonged experimental procedures), he may not be returned to a cage with other male mice
- Male mice are often aggressive and are not considered social
-
RATS:
- Male rats are usually compatible if reared together
- Unfamiliar adult males should not be combined without due caution and observation
- Male rats are usually compatible if reared together
-
HAMSTERS:
- Sexually mature female hamsters are aggressive to cage mates and should be housed singly
- Male hamsters raised together may be compatible
- Unfamiliar adult males may be aggressive and should not be combined without due caution and observation
Last Reviewed by the IACUC 2/08/2023
Soft-Feed and Oral Hydration Support Options (Informational Sheet)
OAR Informational Sheet - Soft-Feed and Oral Hydration Support Options
Informational Sheet: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. Informational Sheets provide information about frequently asked questions and represents guidance for best practices. Deviation from the recommendation(s) does not require specific justification.
Purpose: The purpose of this document is to provide labs with adequate information needed to supplement mice with NapaNectar or Diet Gel.
Some mice require additional nutritional or hydration support, especially small new weanlings, debilitated mice, paralysis models, post-operative mice, craniofacial abnormality mice or others that have challenges in reaching the water valve or feed.
OAR encourages the use of two products: NapaNectar and Diet Gel 31M. Information about these products is summarized below. Note: DietGel 31M is formulated in the same way as our “standard” diet: https://www.envigo.com/resources/data-sheets/7013-datasheet-0915.pdf [25]
Uses for these products:
-
- As part of routine post-operative care or phenotype management as described in the Animal Protocol
- If you need assistance with this, please email iacuc@uiowa.edu [26].
- As part of the care of new weanlings in accordance with new weanling guidance. See https://animal.research.uiowa.edu/investigator-guidance-monitoring-and-managing-new-weanling-mice [23]
- Labs should obtain their own supply and describe it in the Animal Protocol if mouse strains consistently need this support
- As part of routine post-operative care or phenotype management as described in the Animal Protocol
- In consultation with an OAR veterinarian on a case-by-case basis for cage level treatment of animal health issues
If you need assistance in obtaining or using these products, please contact your facility supervisor, veterinary technician or email OAR-Veterinarian@uiowa.edu [27]. These products are stored at room temperature.
|
Details by product |
NapaNectar |
DietGel 31M |
|---|---|---|
|
Support provided |
Gel-like sterile water source |
Softened, complete nutrition, formulated similar to 7913 (OAR standard diet) |
|
Mouse condition |
Needs additional hydration |
Small weanling, debilitated mouse, paralysis, post-operative recovery, etc. |
|
Mice on special diet need additional hydration? |
Use this! |
Don’t use this. |
|
How much to feed? |
½ bag for up to 3-5 mice |
1 cup for up to 5 mice |
|
How often to check mice after placement? |
Check mice daily or more frequently per approved AP |
Check mice daily or more frequently per approved AP |
|
How often to replace? |
As needed, usually every 3-4 days |
As needed, usually every 3-4 days |
|
Cost per packet (UIowa drug order) |
$3.20 per pouch* |
$2.09 per cup* |
|
Cost per case (if purchasing directly) |
$123.08 for case of 50* |
$154.00 for case of 96* |
*As of 11/15/2024. Pricing subject to change.
How to order at U of Iowa:
Go to https://animal.research.uiowa.edu/complete-drug-list-order-form [28]
Select either DIETGEL31 - 2oz cup or NAPA NECTAR 4 oz pouch
How to order a full case:
https://www.selabgroup.net/napa-nectar [29] or www.fishersci.com [30]
https://clearh2o.com/collections/dietgel [31]

*Accessible Word document version of the outlined product details for NapaNectar and DietGel.
Last Reviewed by the IACUC 11/13/2024
Sterilization - Accepted Methods & Monitoring (IACUC Guideline)
Accepted Sterilization Methods & Monitoring
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose
The purpose of these guidelines are to provide guidance on commonly used methods for sterilization of surgical instruments and other materials for use in IACUC-approved Animal Protocols along with methods for monitoring the sterilization procedure.
Accepted Methods for Full Sterilization
- Preferred
- Autoclave (High pressure/temperature)
- Other
- Ethylene oxide gas - for items that cannot withstand high temperature
- Chemical/Cold Sterilization – for items that cannot be sterilized by other methods
Accepted Method for Re-sterilization Between Animals Using Rodent Aseptic Tip Technique
- Dry heat (dry bead sterilizer)
- NOTE: Instruments MUST be fully sterilized by another method between surgical sessions
Autoclave Sterilization
- Autoclave utilizes steam at high heat and pressure which must penetrate the pack to attain sterilization
- Materials such as muslin cloth and crepe paper drape material allow the steam to penetrate into the pack
- Materials such as aluminum foil and wax paper should not be used due to the steam's inability to penetrate them
- Exposure time in an autoclave will vary based on the type of autoclave
- For gravity displacement sterilizers, typically need exposure at 121°C (250°F) for at least 30 minutes.
- For dynamic-air-removal sterilizers, typically need exposure at 132°C (270°F) for at least 4 minutes. [1]
- Bigger packs require more time to reach appropriate heat and pressure levels
- Autoclaved pack should be stored in a dry, dust-free, well-ventilated area, preferably in a closed cabinet
- The length of storage time is indefinite if the pack is stored properly (cabinet, drawer) and does not get wet, torn open or have some other event that compromises its integrity and sterility
- Monitoring
- Autoclave sterilization must be monitored routinely for effectiveness of the sterilization procedure
- Shared laboratory autoclaves may be monitored by each lab or have a designated responsible individual(s)
- Within each pack, place an integrator strip to indicate exposure to the sterilization process
- Packs should be sealed with autoclave tape
- At least once a month, use a Type 5 integrating indicator to monitor the critical variables in the sterilization process
- Type 5 integrating indicators monitor all critical variables in the sterilization process and have stated values that meet or exceed performance requirements in the ISO 11138 series for biological indicators[2],[3]
- Alternatively, Type 5 integrating indicators may be used in place of the routine integrator strip inside each pack
- Examples of Type 5 Integrating Indicators:

-
- At least once a year, use a biological indicator to monitor the effectiveness of microbial killing
- Biological indicators use resistant spores to monitor the lethality of the sterilization process[4]
- Requires either incubation or use of a reader to determine results
- May read and document results on your own or partner with OAR (fee associated with service) to do so
- As an example, OAR currently uses the 3M Attest Rapid Readout Biological Indicator. This requires the use of a specialized machine to read results.
- At least once a year, use a biological indicator to monitor the effectiveness of microbial killing

-
- Documentation
- Integrator strip in packs
- No documentation needed
- Monthly Type 5 integrator strip (if not used in every pack)
- Keep a log near the autoclave or as part of the surgical records
- The log will be reviewed during semi-annual inspections
- Yearly biological indicator
- If partner with OAR, OAR will keep a log of the results
- Otherwise, the lab is responsible for keeping a log
- The log will be reviewed during semi-annual surgical site inspections
- If partner with OAR, OAR will keep a log of the results
- Integrator strip in packs
- Documentation
Ethylene Oxide Sterilization[5]
- NOTE: UIHC Central Sterilizing Services no longer performs ethylene oxide sterilization and it is not provided elsewhere on campus[6],[7]
- Use of an external vendor would be necessary to sterilize by this method
- Used for heat and/or moisture sensitive items
- Effectiveness is dependent on gas concentration, temperature, relative humidity and exposure time
- Items can be sterilized in their final packaging, but effectiveness depends on ability of the gas to freely diffuse through it
- Requires aeration to desorb ethylene oxide from chamber and items
- Some materials, such as certain biocompatible polymers and gels, may take weeks to off-gas the chemical completely.
- Placement of any materials without sufficient time and aeration to desorb may lead to tissue reaction and health concerns
- Disadvantages
- Hazard concerns – flammable, explosive, toxic and carcinogenic
- Length of cycle time
- Cost
Chemical/Cold Sterilization
- Examples of common commercial sterilants and their active ingredient(s):
- NOTE: This information is based on currently available information as of 01/07/2025. Refer to the manufacturer’s instructions for how to appropriately prepare and use.
- Glutaraldehyde solutions[8] (2.4-3.4% depending on product)
-
- Brand examples: CIDEX, Metricide, Omnicide, others
- 10 hours of exposure required for sterilization at room temperature
-
- Sporox Sterilizing and Disinfection Solution (7.5% hydrogen peroxide)
- 6 hours of exposure required for sterilization at room temperature
- Spor-Klenz®: Hydrogen peroxide and peroxyacetic acid
- Sporicidin® Sterilizing and Disinfecting Solution[11]: glutaraldehyde, phenol, phenate
- Active for 14 days once prepared
- 12 hours of exposure required for sterilization at room temperature
- Discretion is required in using these agents to assure that they are used with appropriate safety precautions and that the items being sterilized are compatible with the sterilant
- Factors for effective and proper use of cold sterilization:
- Chemicals must be classified as “sterilants”
- Commonly used disinfectants such as alcohol, iodophors, quaternary ammonium and phenolic compounds are not effective sterilants and are not acceptable for use on items (e.g., catheters, instruments) intended to be sterile
- Physical properties of the items being sterilized must be smooth and impervious to moisture
- All surfaces, both interior and exterior, must be exposed to the sterilant
- Sterilant solution must be clean and fresh
- Date of preparation must be labeled on container
- Chemically sterilized instruments must be thoroughly rinsed both inside and out with sterile saline or sterile water prior to use to avoid tissue damage.
- Instruments must be handled in an aseptic manner to maintain sterility (e.g. handle with sterile gloves and place on a sterile field)
- Chemicals must be classified as “sterilants”
- Standard Operating Procedures (SOP) MUST be posted in the laboratory when using chemical sterilization methods and must contain the following information:
- Agent used (i.e. active ingredient)
- How sterilant is prepared
- How long sterilant is active once prepared (expiration time)
- Exposure time required for sterilization of instruments/supplies
- How the sterilant is removed prior to use in an aseptic technique
- Template and an example SOP may be found here: [33]Chemical Sterilization Standard Operating Procedures (SOPs) [34]
- Consult with an OAR veterinarian if there are any questions relating to the use of chemical/cold sterilants.
Dry Bead Sterilization
- Used to sterilize the tips of surgical instruments in between multiple surgeries
- Instruments MUST be fully sterilized by another method between surgical sessions
- A surgical session includes those surgeries performed on the same day
- Sterilizer must be activated for a minimum of 20 minutes to reach the appropriate temperature before being used[12]
- All biological debris (e.g. blood, tissue) must be removed before placing the instruments into the sterilizer
- Instruments must be inserted into the sterilizer for a minimum of 15 seconds before sterilization is attained[13]
- Once the instruments are removed from the sterilizer, the tips will be VERY HOT
- They must be allowed to cool before using to avoid burning the animal
- Only the tips of the instruments are sterilized and the handles are considered to be contaminated
- The instruments must be utilized in a fashion that the tips of the instruments remain sterile. See details regarding aseptic tip technique in Rodent Survival Surgery Guidelines [5]
[1] https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/steam-sterilization.html [35]
[3] https://www.sterislifesciences.com/resources/documents/article-reprints/chemical-indicators-for-steam-sterilization [37]
[4]https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/ste... [38]
[5] https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/ethylene-oxide-sterilization.html [39]
[6] https://medcom.uiowa.edu/theloop/announcements/faqs-central-sterilizing-services-new-facility-to-open-spring-2020 [40]
[7] https://medcom.uiowa.edu/theloop/announcements/central-sterilizing-services-facility-update-instrument-processing-care-and-handling [41]
[8] https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices-information-manufacturers/fda-cleared-sterilants-and-high-level-disinfectants-general-claims-processing-reusable-medical-and [42]
[9] https://www.sterislifesciences.com/products/surface-disinfectants/sporicide-cleaners-and-sterilant/spor-klenz-concentrate-cold-sterilant [43]
[10] https://www.sterislifesciences.com/products/surface-disinfectants/sporicide-cleaners-and-sterilant/spor-klenz-ready-to-use-cold-sterilant [44]
[11] https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices-information-manufacturers/fda-cleared-sterilants-and-high-level-disinfectants-general-claims-processing-reusable-medical-and [42]
[13] https://www.harvardapparatus.com/hot-bead-dry-sterilizers.html [45]
Last Reviewed by the IACUC 1/09/2025
Substance Administration - Recommended Volumes (Informational Sheet)
Recommended Volumes for Administered Substances (Informational Sheet)
Informational Sheet: The Office of Animal Resources has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. This Informational Sheet provides the current guidance on recommended testing of research biologics for pathogens.
Purpose:
This document provides information about administration volumes and needle sizes for commonly employed routes of fluid administration in various animal species. The volume limits are consensus figures based on published literature and are set up to maintain animal welfare and prevent potential complications, such as muscle damage with high volume intramuscular injection, or aspiration & pulmonary injury with high volume oral administration.
Procedures:
- Give injections at a constant flow rate
- No resistance should be encountered during injection
- Do not apply overt pressure on the syringe’s plunger
- See below for maximum recommended volume and needle size
- Compare the weight in the table to the weight of the animal being used in the procedure and alter dose accordingly
- Max limit with oil-based vehicles should be half the listed max limit and may require a larger gavage needle
- Always use the smallest needle size (largest gauge) that is applicable to the procedure
Precautions:
- Do not inject into inflamed or damaged tissue unless medically indicated
- Injecting relatively large volumes of solutions that are below body temperature may lead to hypothermia
- Inject separate drugs/compounds at different sites to avoid cross reaction of chemicals
- Limit subcutaneous administration to 2-3 sites per day
- Limit intramuscular administration to 2 sites at one time
- Intravenous volumes listed are for slow injection over 3-10 minutes. Bolus volumes should be less, typically 10-50% of the slow injection volume.
- Exceptions apply when medically indicated or scientifically justified, consult with Office of Animal Resources (OAR) veterinary staff for guidance
Recommended Maximum Volumes and Needle Sizes per Route, per Site, and per Species
|
Species & weight |
Per os (oral) |
Subcutaneous |
Intramuscular |
Intraperitoneal |
Intravenous |
|---|---|---|---|---|---|
|
Rat 200g |
4 ml 20 ml/kg 16 G |
1 ml 5 ml/kg 20 G |
0.2 ml total/site - 21 G |
4 ml 20 ml/kg 21 G |
4 ml 20 ml/kg 23 G |
|
Mouse 25g |
0.5 ml 20 ml/kg 18 G |
0.25 ml 10 ml/kg 20 G |
0.1 ml total/site - 23 G |
0.5 ml 20 ml/kg 21 G |
0.625 ml 25 ml/kg 25 G |
|
Hamster 100g |
2 ml 20 ml/kg 18 G |
0.5 ml 5 ml/kg 20 G |
0.2 ml total/site - 21 G |
2 ml 20 ml/kg 21 G |
2 ml 20 ml/kg 25 G |
|
Guinea pig 800g |
16 ml 20 ml/kg |
4 ml 5 ml/kg 20 G |
0.2 ml total/site - 21 G |
16 ml 20 ml/kg 21 G |
4 ml 5 ml/kg 23 G |
|
Rabbit 3kg |
60 ml 20 ml/kg
|
15 ml 5 ml/kg 20 G |
1 ml 0.5 ml/kg up to max 1 ml 20 G |
30 ml 10 ml/kg 20 G |
30 ml 10 ml/kg 21 G |
|
Cat 5kg |
75 ml 15 ml/kg |
25 ml 5 ml/kg 20 G |
1 ml 0.5 ml/kg up to max 1 ml 20 G |
100 ml 20 ml/kg 20 G |
50 ml 10 ml/kg 21 G |
|
Dog 10kg |
150 ml 15 ml/kg |
10 ml 1 ml/kg 20 G |
3 ml 0.5 ml/kg up to max 3 ml 20 G |
200 ml 20 ml/kg 20 G |
100 ml 10 ml/kg 20 G |
|
Pig 25kg |
375 ml 15 ml/kg |
37.5 ml 1.5 ml/kg 20 G |
5 ml 0.5 ml/kg up to max 5 ml 20 G |
500 ml 20 ml/kg 20 G |
250 ml 10 ml/kg 18 G |
|
Ferret 1kg |
15 ml 15 ml/kg
|
5 ml 5 ml/kg 21 G |
1 ml 0.5 ml/kg up to max 1 ml 23 G |
20 ml 20 ml/kg 21 G |
10 ml 10 ml/kg 21 G |
|
Goat/Sheep 45kg |
900 ml 20 ml/kg
|
225 ml 5 ml/kg 19 G |
5 ml total/site - 18 G |
450 ml 10 ml/kg 19 G |
450 ml 10 ml/kg 18 G |
Last Reviewed by the IACUC 6/14/2023
Substance Administration - Use of Drugs and Chemicals in Laboratory Animals (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose:
These guidelines describe the use of drugs and other chemicals administered to laboratory animals at the University of Iowa. “ The NIH Office of Laboratory Animal Welfare (OLAW) and the United States Department of Agriculture (USDA) both have determined that the use of non-pharmaceutical-grade compounds should be based on (1) scientific necessity, (2) non-availability of an acceptable veterinary or human pharmaceutical-grade compound, and (3) specific review and approval by the institutional ACUC.”1 The use of drugs falls into one of two usage categories. These guidelines apply to drugs used for experiments or for therapeutic purposes. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
The use of non-pharmaceutical grade compounds in animals may be necessary and appropriate, but must be scientifically justified in an IACUC-approved Animal Protocol.
Definitions
- FDA – Food and Drug Administration
- USP/NF – United States Pharmacopeia/National Formulary
- BP – British Pharmacopeia
- Pharmaceutical grade compound: Drug, biologic, or reagent which is approved by the FDA or for which a chemical purity standard has been established by USP/NF or BP.
- Phosphate buffered saline (PBS) if tissue culture grade and sterile
- Non-pharmaceutical grade compound: Any substance which does not meet the above definition of pharmaceutical grade, including:
- Analytical grade bulk chemical: ~99% purity chemical, Certificate of Analysis typically available
- Pharmaceutical grade drug compounded with non-pharmaceutical grade vehicle or other substance
Drugs Administered for Therapeutic Purposes
- Current standards for the veterinary therapeutic care of research animals state that pharmaceutical grade medications should be used for routine medical treatment
- Examples of therapeutic purposes include:
- Sedation and anesthesia for surgery or other procedures
- Relief or treatment of disease or injury
- Pain control (analgesia)
- Euthanasia
- Drugs used for veterinary care, either as part of an IACUC-approved Animal Protocol or an OAR veterinarian-approved treatment plan, should be obtained from a veterinary supply or from a pharmaceutical supplier licensed by the FDA, if available from such sources
Substances Administered for Experimental Purposes2
When developing a proposal to administer a substance to an animal, the following factors should be considered:
- Purity/grade
- The following order of choice should be applied:
- FDA-approved veterinary or human pharmaceutical compound
- FDA-approved pharmaceutical compound used to compound a needed dosage/formulation
- USP/NF or BP pharmaceutical grade compound used to compound a needed dosage/formulation
- Analytical grade bulk chemical used to compound a needed dosage/formulation
- Other grades and sources of compounds
- The following order of choice should be applied:
- Safety
- Efficacy
- Sterility
- Pyrogenicity
- Stability
- pH/osmolality
- Site/route of administration
- Pharmacokinetics
- Physiological compatibility
- Quality control
The following questions should be considered when deciding what formulation(s) to use for your animal experiments:
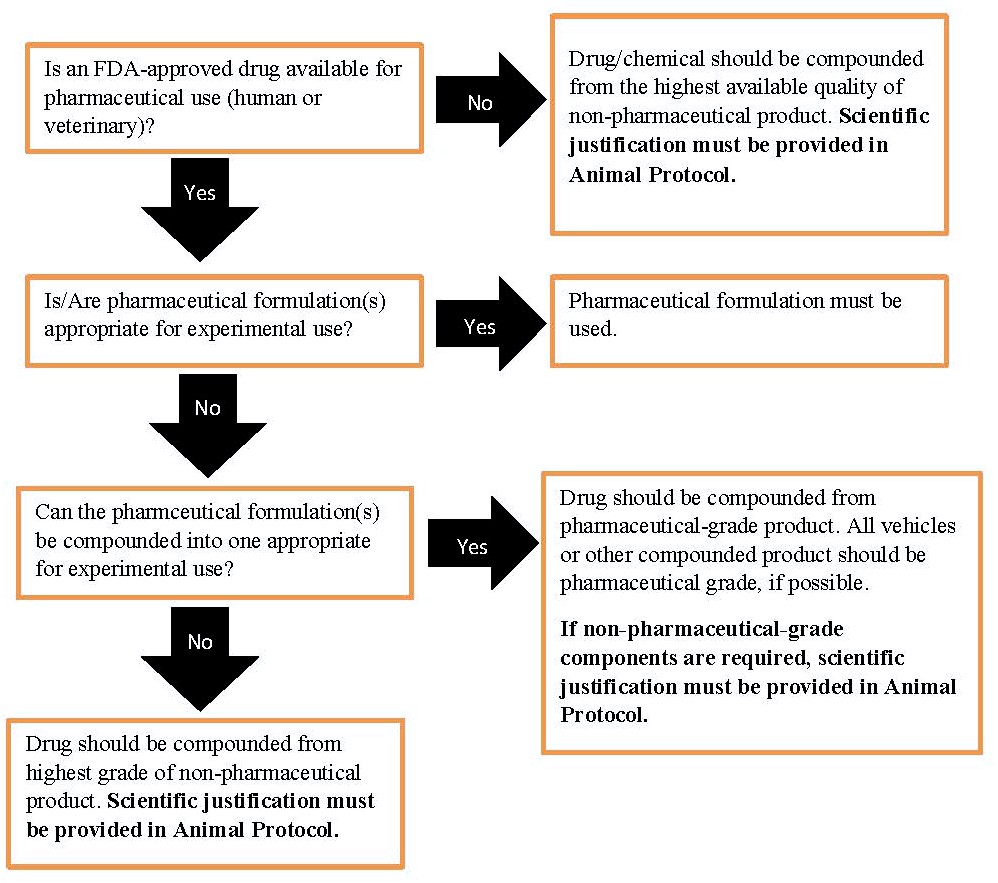
Scientific Justification
- In situations where use of a non-pharmaceutical grade substance is necessary and appropriate, the following sample text may be used and/or modified to illustrate scientific justification in your Animal Protocol:
- No pharmaceutical-grade human or veterinary drug is available
- A pharmaceutical-grade drug is available, but is not compatible with the concentration, formulation, delivery, or vehicle requirements of experimental administration
- A pharmaceutical-grade drug is available, but the use of a non-pharmaceutical grade product is required to replicate methods from previous studies because results must be directly compared to the results of replicated studies
Anesthetic Agents for Aquatic Species
The most commonly used anesthetic agent for fish and frogs in our facilities is tricaine methanesulfonate, or MS-222. This agent is available as an FDA-approved veterinary drug under the labels Finquel or Tricaine-S. As a result, we recommend the use of these veterinary pharmaceutical-grade products according to the product label, which involves dilution in the appropriate aquatic habitat water from the housing facility, followed by buffering with sodium bicarbonate to an appropriate pH. Benzocaine may also be used. Dosage and storage instructions contained in the IACUC Guidelines on Anesthesia should be followed, unless otherwise described and justified in the Animal Protocol.
Preparation and Handling of Drugs/Chemical Agents
- Agents to be administered to animals must be handled and stored so as to maintain sterility and efficacy
- Appropriate closed sterile containers (e.g. injection vials, red-topped blood tubes) must be used, rather than snap-cap or screw-top containers
- The smallest amount of agent suspension/dilution/mixture should be used to minimize storage time prior to administration
- The rubber injection port/cap should be swabbed with alcohol prior to insertion of the needle
- Use a clean, sterile container for each preparation (do not reuse)
- Use new sterile needles for each entry into a sterile container
- Maintain needles in a sterile manner prior to injection
- Do not rest needles on non-sterile surfaces
- The re-use of needles for multiple animals is strongly discouraged due to loss of sharpness and biosecurity/cross-contamination concerns
- Examine multiple-dose injection vials/tubes prior to use for evidence of physical or chemical contamination
- Discard any substance meeting any of the following criteria:
- Particulate matter
- Precipitation of solids
- Turbid or discolored appearance
- Mislabeled or unlabeled container
- Damage to the rubber stopper compromising integrity
- Discard any substance meeting any of the following criteria:
- All containers must be labeled with:
- Name of the drug(s)/chemical(s) contained
- Concentration of the drug/chemical
- Date of expiration (see below)
Expiration of Drug Dilutions/Mixtures
- Sterile dilutions or mixtures of drugs may result in a shorter effective expiration date than the expiration date of the individual components, due to risk of contamination and dilution of preservatives
- An expiration date of six (6) months from the date of preparation, or the earliest expiration date for any single component (if less than six months), is recommended unless scientifically justified otherwise3
Expired Drugs
- Expired drugs must not be administered to any animal without explicit IACUC approval
- All expired drugs, including anesthetics and analgesics, must be segregated and clearly mark “EXPIRED”
- For information on storage and destruction of expired DEA controlled substances, please refer to the controlled substances link below
DEA Controlled Substances
References:
1) "Guidelines for the Use of Non-Pharmaceutical Grade Compounds in Laboratory Animals." Oacu.od.nih.gov. 8 Jan. 2010.
2) OLAW FAQ F.4 [47] Accessed 2/11/13.
3) Beyond-use Dating of Extemporaneously Compounded Ketamine, Acepromazine, and Xylazine: Safety, Stability, and Efficacy over Time. JAALAS 48.6 (2009): 718-26.
Last Reviewed By the IACUC 11/8/2023
Surgery - Non-Survival Surgery (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Non-Survival Surgery
Purpose: The purpose of these guidelines is to provide direction for personnel conducting non-survival surgery on research animals at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Instrument and Equipment Preparation
- All instruments and materials to be used should be visibly clean at the beginning of surgery
- Expired surgical materials such as suture or implants may be used
Medications
- All drugs (anesthetics, analgesics etc.) and any fluids administered must not be expired
Surgery Location
- Allocate a clean uncluttered work area away from laboratory traffic, ventilation ducts and open windows
- Dedicate the area solely to surgical procedure(s) when in use
Area Preparation
- Clean the work surface of visible dirt or debris
- Apply a clean drape over the working surface where the surgery will be performed
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia [48] for details of requirements
- For prolonged procedures, apply ophthalmic ointment to both eyes to prevent corneal desiccation
- Remove hair from the surgical site using one of the following:
- Clipper blade
- Depilatory cream
- Fur plucking
- Remove loose hair and visible dirt/debris from the surgical site
- Wipe the surgical site clean using alcohol, surgical scrub or warm water
- Avoid excessive wetting of non-surgical areas of the animal with alcohol or disinfectant as this can exacerbate hypothermia
Surgeon Preparation
- At a minimum, put on a clean lab coat or scrub top and a clean pair of gloves
- Additional personal protective equipment may be needed depending on other factors, such as the animal species or type of procedure
Euthanasia
- At the end of the procedure, euthanize the animal under anesthesia in accordance with the approved Animal Protocol.
- Refer to the IACUC Guidelines on Euthanasia [16] for further details
Record Keeping
- Record the following information in addition to the anesthesia record requirements [49] Note: the anesthesia record may be combined with the surgical record; click here for the template. [50]
- Procedure
- Time of euthanasia
- Keep surgery and anesthesia records readily accessible for review
Last Reviewed 11/9/2022
Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to provide direction for personnel conducting survival surgery on research rodents (mice of the genus Mus, rat of the genus Rattus) at the University of Iowa. These guidelines do not apply to USDA-covered species at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures on rodent species (Mus, Rattus), or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Definitions:
Best Practice:
- Technique or method that consistently shows superior results
- Items in Guidelines labeled “best practice” are strongly recommended, but not required
Instrument and Equipment Sterilization
- All materials, implants, and substances used in surgery and/or placed inside the animals must be either sterile single-use (disposable) or sterilized prior to each use
- Start each day using only sterile instruments, surgical supplies and wound closure materials
- If a sterile instrument or sterile glove comes in contact with a non-sterile item, it is NO LONGER STERILE
- Re-sterilize or replace contaminated items before continuing aseptic procedures
- Re-sterilize or replace items before continuing aseptic procedures between animals
- Refer to sterilization methods [51]for further information on material sterilization methods
- Sterile suture material and/or mechanical wound closures must be appropriate to the procedure and purpose
- Please consult an OAR veterinarian for guidance if you are unsure which closure material/method is appropriate for your purpose
- Synthetic non-absorbable monofilament suture (e.g. Prolene) is typically recommended for skin closures
- Silk sutures and other braided materials are undesirable for skin closures due to risk of wicking surface bacteria into the tissues. Use of silk sutures for skin closures must be described and scientifically justified in the Animal Protocol.
Surgery Location
- Allocate a clean uncluttered work area away from laboratory traffic, ventilation ducts and open windows
- Dedicate the area solely to surgical procedure(s) when in use
- Utilize a separate room used primarily for aseptic procedures, if possible
Area Preparation
- Clean work surface with a disinfectant
- Apply a clean drape over the working surface where the surgery will be performed
- Establish a sterile field near the animal for placement of sterile instruments (see Figure 1)
Figure 1: Surgical Area Preparation
A) Common supplies for survival rodent surgery:
A) Common supplies for survival rodent surgery: disinfectant wipes or spray, hot bead sterilizer, two pairs of sterile gloves, a clean drape (the surgical area), several sterile cotton-tipped applicators for sterile preparation of the surgical site and hemostasis, povidine-iodine or chlorhexidine scrub and 70% ethanol, sterile saline, insulin syringes, a scalpel blade, and a sterile surgical pack and instrument stand.
B) Sterile-tip instrument set-up
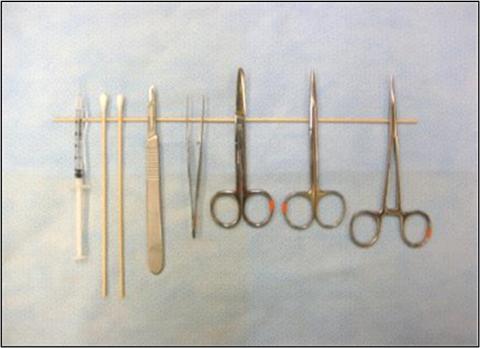
B) Sterile-tip Instrument Setup - Instruments are positioned with the hinge resting on the instrument stand (sterile wooden dowel, or other sterile dividing material), which divides the underlying drape into upper STERILE and lower NON-STERILE areas. This setup ensures maintenance of instrument tip sterility in spite of contaminated handles when using sterile “instrument tip” technique. The use of an instrument stand maintains the instrument tips at an elevated position (insert), reducing surface contact and promoting sterility.
C) Surgical Set-up
C) Surgical Setup - The animal is positioned on the surgical field (with heat support) and draped. Instruments are placed on the instrument drape in an organized fashion, utilizing the instrument stand.
Examples of hard surface disinfectants
| AGENT | EXAMPLES | COMMENTS |
|---|---|---|
| Quaternary Ammonium | Sani Cloth®, Roccal®, Quatricide®, Tec-Surf II® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorine | Sodium hypochlorite (Clorox® 10% solution) Chlorine dioxide (Clidox®, MB-10®) |
Remove organic matter prior to disinfection (organic matter reduces activity). Note: Solutions need to be made up fresh daily to maintain activity. |
| Glutaraldehydes | Glutaraldehydes (Cetylcide®, Cide Wipes®) |
Remove organic matter prior to disinfection (organic matter reduces activity). |
| Phenolics | Lysol®, TBQ® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorhexidine | Nolvasan® , Hibiclens® | Remove organic matter prior to disinfection (organic matter reduces activity). |
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia [48] for details of anesthesia agents, procedures, and monitoring requirements
- Administer appropriate analgesia at the time of anesthetic induction
-
- Refer to the IACUC Guidelines on Analgesia [52] for further details
- Apply ophthalmic ointment to both eyes to prevent corneal desiccation
- Remove hair from the surgical site using one of the following:
- Clipper blade
- Depilatory cream
- Fur plucking
- Remove loose hair and visible dirt/debris from the surgical site
- Perform a surgical site preparation of the incision site.
- Note: alcohol alone is not sufficient for surgical preparation
- Utilize povidone-iodine ("betadine") or chlorhexidine antiseptic products for the surgical site preparation
- Available in two forms:
- "Solutions" which contain the antiseptic agent alone
- "Surgical scrubs" which contain a cleansing agent combined with the antiseptic agent
- Properly diluted antiseptic solutions may be left on the skin during surgery but surgical scrubs can be irritating and must be rinsed away after use
- Available in two forms:
- Apply betadine or chlorhexidine surgical scrub or solution with clean gauze in a circular fashion starting at the surgical incision site and rotating outward
- Alternate surgical scrub or solution with 70% alcohol or sterile saline (best practice but not required unless using surgical scrub)
- Repeat a minimum of three times discarding cotton pad or swab after each use
- Apply betadine, chlorhexidine solution (NOT scrub), or end with a final application of alcohol or sterile saline after the surgical prep
- Avoid excessive wetting of non-surgical areas of the animal with alcohol or antiseptic as this can exacerbate hypothermia
- Cover rodent with sterile drape or Glad Press and Seal to avoid contamination of the incision, instruments and supplies
- It is recommended to use clear drapes to facilitate observation of the rodent
Optimal Surgical Prep Procedure:
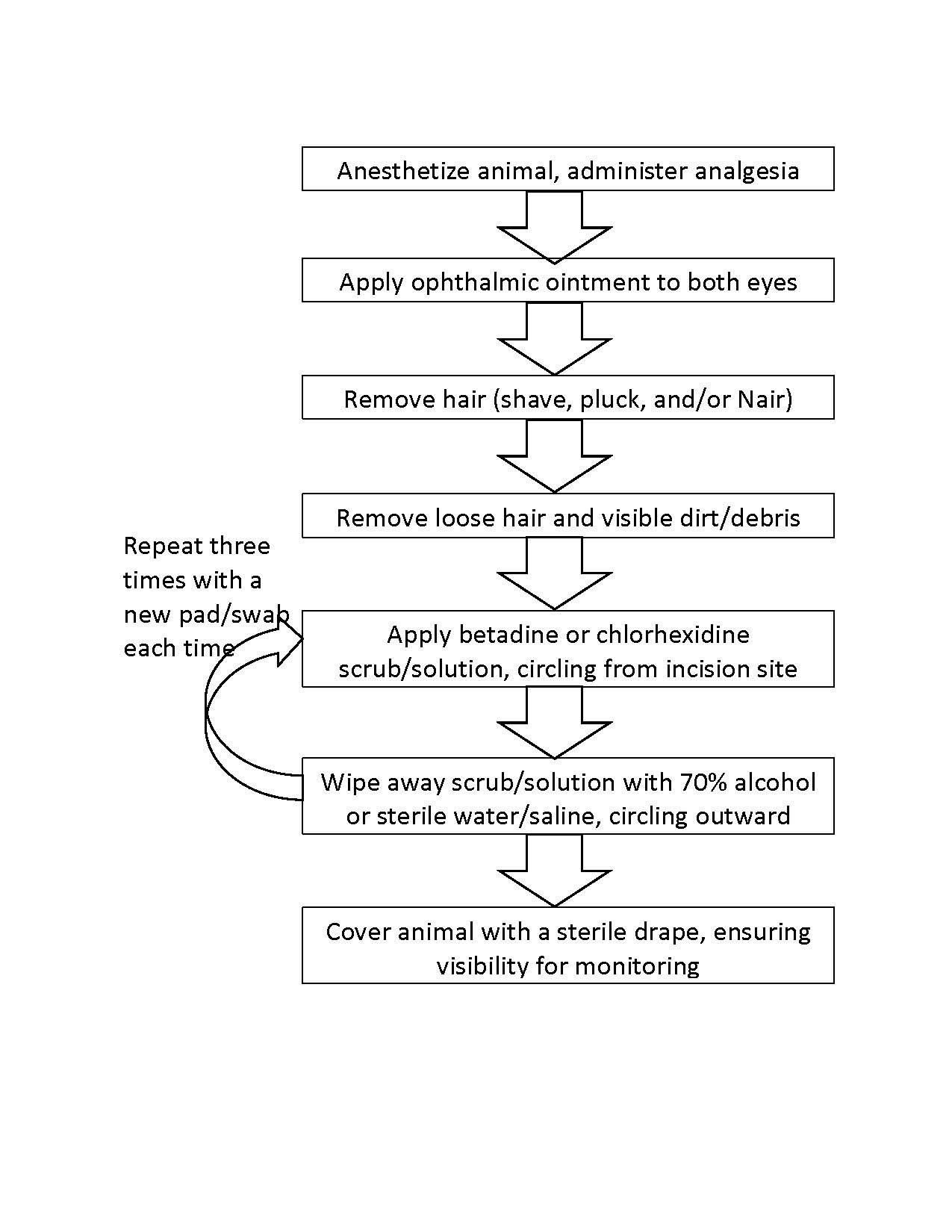
Aseptic Surgical Technique
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Unwrap sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging or instruments as this will compromise the sterility of the instruments
- Perform a surgical scrub of the surgeon’s hands
- Put on sterile gloves without touching the exterior of the glove surface
- Use the sterile interior of the glove packaging as a sterile field for instruments
- Maintain sterility of gloves and instruments throughout the surgery
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Aseptic Tip Technique
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Start with sterile instruments at the beginning of each set of distinct surgical procedures
- Unwrap sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging or instruments as this will compromise the sterility of the instruments
- Wash hands or perform surgical scrub
- Put on sterile or procedural gloves
- The interior of sterile glove packaging may be used as a sterile field for instruments if sterile gloves are used
- Arrange the sterile instruments so that the tips are within a sterile field and the handles are outside the sterile field
- Do not contaminate the sterile tips of the instruments during this process
- Instrument tips must be maintained within this sterile field throughout the surgery
- Utilize only the sterile tips of the instruments inside the body cavity
- Tips of surgical instruments must be sterilized between animals or multiple surgeries. (see Figure 1)
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Postsurgical Care and Monitoring
- Immediately post-operatively, evaluate the animal’s surgical blood loss and provide replacement fluids if appropriate. (See Informational Sheet [53] for estimation guidance)
- Commercial (pharmaceutical grade) sterile saline for injection (NaCl) or lactated ringer’s solution may be given subcutaneously in a volume equal to the estimated blood loss.
- Recovery the animal from anesthesia according to the IACUC Guidelines on Anesthesia [48]
- Assess the animals at least daily (including weekends and holidays) for at least five days post-operatively (days after the surgery) or as justified in the Animal Protocol
-
- Example 1, Surgical procedure occurs on Monday (day 0), the animals must be monitored at least daily Tuesday (day 1 post-op) through Saturday (day 5 post-op)
- Example 2, Surgical procedure occurs on Wednesday (day 0), the animals must be monitored at least daily Thursday (day 1 post-op) through Monday (day 5 post-op)
- Monitor animals for signs of pain Pain Recognition by Species [54] and contact OAR Veterinary Staff if signs are observed
- Monitor incision site for the following:
- Incisional integrity (i.e. sutures intact and wound is closed)
- Incision site infection: Heat, excessive swelling or purulent discharge
- Excessively tight sutures
- Consult with Office of Animal Resources Veterinary staff if above signs or other problems arise
- Including potential re-closure of surgical site dehiscence/wound closure failure
- Remove wound closure 7-14 days post-operatively
Record Keeping
- Record the following information in addition to the anesthesia record requirements [49]
- Note: the anesthesia record may be combined with the surgical record; a template can be found here [4]
- Animal Protocol number, PI name, individual performing procedure
- Procedure
- Date, time, dose and route of each analgesia administration
- Date when wound closures (sutures, staples, clips) are removed/are no longer present
- Post-operative monitoring observations, including the date and time of each observation and a brief description of the animal’s health status and incision site appearance
- Reflect the animal’s health status by commenting on animal’s appearance, posture and activity.
- Keep surgery, anesthesia and post-operative records readily accessible for review
Last reviewed by the IACUC: 10/11/2023
Surgery - Rodent Blood Loss (Informational Sheet)
Informational Sheet: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. Informational Sheets provide information about frequently asked questions and represents guidance for best practices. Deviation from the recommendation(s) does not require specific justification.
Purpose: The purpose of this document is to provide guidance on estimating surgical blood loss for use in IACUC-approved Animal Protocols.
An animal’s total blood loss should be estimated post-operatively.
- No more than 1% of an animal’s body weight should be lost in a 24 hour period (200g Rat is 2ml of blood).
- If blood loss exceeds 1% of an animal’s body weight, replacement fluids should be given and OAR veterinary staff should be contacted.
- When total fluid loss is estimated after surgery an equal volume of commercial (pharmaceutical grade) sterile saline for injection (NaCl), lactated ringer’s solution, or other physiologic appropriate sterile fluid may be given subcutaneously.
- Subcutaneous injections can be administered with a syringe in areas of loose skin i.e., the interscapular region.
Refer to Table 1 for the blood volumes associated with saturation of common hemostatic tools used in rodent surgery. As an example, 25 saturated cotton applicator tips would exceed the blood loss limit of a 200g rat.
Table 1: Blood volumes associated with saturation of common hemostatic tools used in rodent surgery

Last reviewed by the IACUC: 2/14/2024
Surgery - USDA Covered Species Survival Surgery (Guideline)
Survival Surgery of USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Non-Rodent Mammal Survival Surgery
Purpose: The purpose of these guidelines is to provide direction for personnel conducting survival surgery on research mammals (other than Mus or Rattus) at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures on USDA covered species, or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Definitions
- Major Survival Surgery: Any surgical intervention that penetrates and exposes a body cavity or produces permanent impairment of physical or physiologic functions
- Minor Survival Surgery: Survival surgical intervention(s) that do(es) not expose a body cavity and/or causes little or no physical impairment
- Non-Survival Surgery: Surgical intervention(s) that are completed under a surgical plane of anesthesia from which the animal does not recover prior to euthanasia
Instrument and Equipment Sterilization
- Use only sterile instruments, surgical supplies and wound closure materials
- If a sterile instrument or sterile glove comes in contact with a non-sterile item, it is NO LONGER STERILE
- Re-sterilize or replace contaminated items before continuing aseptic procedures
- New fully sterilized supplies must be used for each animal
- Refer to sterilization methods [51] for further information on material sterilization methods
Preoperative Considerations
- Acclimate animals to the housing facility prior to surgery (best practice; not required)
- Assess each animal’s health status prior to inducing anesthesia
- Consult with an Office of Animal Resources (OAR) veterinarian for any animal identified to have a health concern via an Orange Card and/or with any questions concerning health status
- Fast animal prior to inducing anesthesia
- Do not fast rabbits or rodents
- Prevent development of bloat in ruminants
- Utilize a 24-48 hour pre-anesthetic fasting period
- Shorten the total anesthetic period when possible
- If rumen distention occurs, immediately consult with an OAR veterinarian so that appropriate treatment may be initiated
Surgery Location
- Perform major survival surgeries in a dedicated IACUC-approved surgical suite
- Minor survival surgeries or non-survival surgeries may be performed in a dedicated suite or as listed on the Animal Protocol
Area Preparation
- Clean and disinfect all surfaces and equipment in the surgical suite with a quaternary ammonium compound or similar disinfectant using appropriate contact time.
- Establish a sterile field near the animal for placement of sterile instruments
Examples of hard surface disinfectants
| AGENT | EXAMPLES | COMMENTS |
|---|---|---|
| Quaternary Ammonium | Sani Cloth®, Roccal®, Quatricide®, Tec-Surf II® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorine | Sodium hypochlorite (Clorox® 10% solution) Chlorine dioxide (Clidox®, MB-10®) |
Remove organic matter prior to disinfection (organic matter reduces activity). Note: Solutions need to be made up fresh daily to maintain activity. |
| Glutaraldehydes | Glutaraldehydes (Cetylcide®, Cide Wipes®) |
Remove organic matter prior to disinfection (organic matter reduces activity). |
| Phenolics | Lysol®, TBQ® | Less affected by organic material than other disinfectants. |
| Chlorhexidine | Nolvasan® , Hibiclens® | Remove organic matter prior to disinfection (organic matter reduces activity). |
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia [48] for details of anesthesia procedures and monitoring requirements
- Administer appropriate analgesia at the time of anesthetic induction
- Refer to the IACUC Guidelines on Analgesia [52] for further details
- Apply ophthalmic ointment to both eyes to prevent corneal desiccation
- Clip hair from the entire surgical field (everything that will not be covered by drapes)
- Remove loose hair and visible dirt/debris from the surgical site
- Move the animal from the prep room to the surgical table and secure the animal to the table
- Perform a surgical scrub of the incision site
- Note: alcohol alone is not sufficient for surgical preparation
- Utilize povidone-iodine ("betadine") or chlorhexidine for the surgical site preparation
- Available in two forms:
- "Solutions" which contain the antiseptic agent alone
- "Surgical scrubs" which contains a cleansing agent combined with the antiseptic agent
- Properly diluted antiseptic solutions may be left on the skin during surgery but surgical scrubs can be irritating and must be rinsed away after use
- Apply betadine or chlorhexidine surgical scrub or solution with clean gauze in a circular fashion starting at the surgical incision site and rotating outward
- Alternate surgical scrub or solution with 70% alcohol or sterile saline (best practice but not required unless using surgical scrub)
- Repeat a minimum of three times discarding cotton pad or swab after each use
- Apply betadine or chlorhexidine solution (NOT scrub) after the surgical prep scrub (best practice)
- Utilize povidone-iodine ("betadine") or chlorhexidine for the surgical site preparation
- Avoid excessive wetting of non-surgical areas of the animal with alcohol or disinfectant as this can exacerbate hypothermia
Optimal Surgical Scrub Prep Procedure:
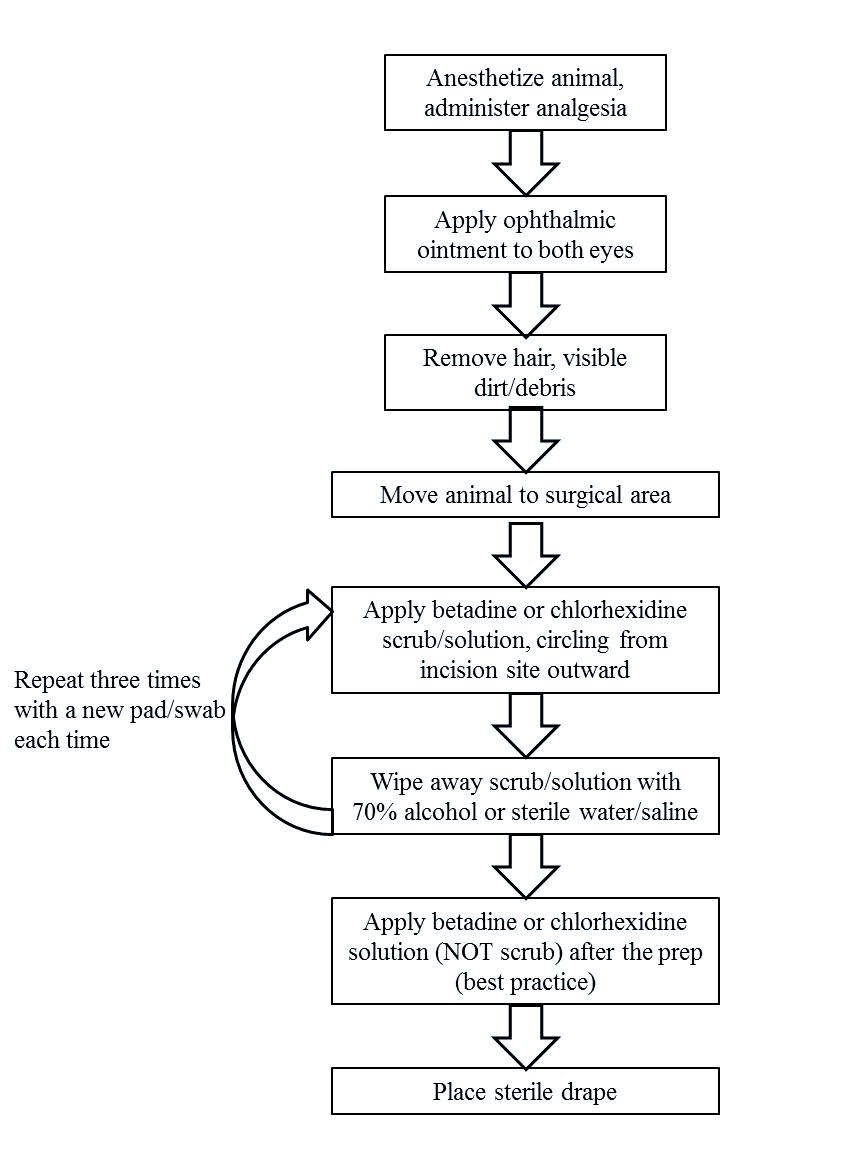
Surgeon Preparation
- Surgeon and surgical assistant:
- Wear clean scrubs, shoe covers, surgical mask, and bonnet
- Perform surgical scrub of hands
- Put on sterile gown and gloves while maintaining sterility
- Observers/anesthetist:
- Wear clean scrubs, shoe covers, surgical mask, bonnet and disposable gloves
Aseptic Surgical Technique
- Place sterile drape on animal to prevent contamination of the incision, instruments and supplies
- No fur should be visible in the surgical field
- Unwrap the sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging (except with sterile gloves) as this will compromise the sterility of the instruments
- Maintain sterility of gloves and instruments throughout the surgery
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Care During Surgery
- Provide fluids (e.g., IV, IP, SQ) to maintain adequate hydration as described in the approved Animal Protocol
- Fluids should be provided during:
- major surgeries lasting longer than 30 minutes
- prolonged anesthesia for minor surgeries (and nonsurgical events, per Anesthesia Guidelines)
- Heat support (warming blanket, heated surgical stage/table, warmed IV fluids, etc.) is recommended for procedures/anesthesia lasting longer than 30 minutes
Postsurgical Care and Monitoring
- Assess the animals at least daily (including weekends and holidays) for at least five days post-operatively (days after surgery)
-
- Example 1, Surgical procedure occurs on Monday (day 0), the animals must be monitored at least daily Tuesday (day 1 post-op) through Saturday (day 5 post-op)
- Example 2, Surgical procedure occurs on Wednesday (day 0), the animals must be monitored at least daily Thursday (day 1 post-op) through Monday (day 5 post-op)
- Monitor animals for signs of pain Pain Recognition by Species [54]:
- Monitor incision site for the following:
- Incisional integrity (i.e. sutures intact and wound is closed)
- Incision site infection: Heat, excessive swelling or purulent discharge
- Excessively tight sutures
- Remove sutures 7-14 days post-operatively
- Consult with OAR Veterinary staff if problems arise
Record Keeping
- Record the following information in addition to the anesthesia record requirements [49]
- Note: the anesthesia record may be combined with the surgical record; a template can be found here [4]
- PI Name, Animal Protocol Number
- Procedure
- Date, time, dose and route of each analgesia administration
- Date when wound closures (sutures, staples, clips) are removed/are no longer present
- Post-operative monitoring observations, including the date and time of each observation and a brief description of the animal’s health status and incision site appearance
- Reflect the animal’s health status by commenting on animal’s attitude, activity, hydration, appetite. For example:
- Attitude:
- Bright, alert and responsive (BAR)
- Quiet, alert and responsive (QAR)
- Depressed
- Activity
- Normal activity
- Decreased activity
- Appetite
- Normal appetite (normal feces, urine)
- Decreased appetite (decreased fecal and urine output?)
- Hydration:
- Normal hydration
- Dehydrated (increased skin tent)
- Attitude:
- Keep surgery, anesthesia and post-operative records readily accessible for review
Last Reviewed by the IACUC 10/11/2023
Surgery - Xenopus Oocyte Harvest (Guideline)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to provide direction for personnel conducting surgery to harvest oocytes from Xenopus frogs at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures on Xenopus, or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Definitions
- Best Practice
- Technique or method that consistently shows superior results
- Items in Guidelines labeled as "best practice" are strongly recommended, but not required
Instrument and Equipment For Surgery
- It is best practice to start each surgery session using sterile instruments, surgical supplies and wound closure materials At a minimum start with clean instruments (free of gross debris) that have had their tips sterilized with a dry bead sterilizer
- It is not recommended to use chemical sterilants for equipment used for frog surgeries
- improperly rinsed instruments can negatively impact the health of the frogs
- If a sterile instrument or sterile glove comes in contact with a non-sterile item, it is NO LONGER STERILE
- Re-sterilize or replace contaminated items before continuing aseptic procedures
- Re-sterilize or replace items before continuing aseptic procedures between animals
- It is not recommended to use chemical sterilants for equipment used for frog surgeries
- Refer to sterilization methods [51] for further information on material sterilization methods
Surgery Location
- Designate a clean uncluttered work area away from laboratory traffic, ventilation ducts and open windows
- Dedicate the area solely to surgical procedure(s) when in use
- Utilize a separate room used primarily for aseptic procedures, if possible
Area Preparation
- Clean work surface with a disinfectant (see table below)
- Apply a clean drape over the working surface where the surgery will be performed
- Establish a sterile field near the animal for placement of sterile instruments
Examples of hard surface disinfectants
|
AGENT |
EXAMPLES |
COMMENTS |
|---|---|---|
|
Quaternary Ammonium |
Sani Cloth®, Roccal®, Quatricide®, Tec-Surf II® |
Remove organic matter prior to disinfection (organic matter reduces activity). |
|
Chlorine |
Sodium hypochlorite |
Remove organic matter prior to disinfection (organic matter reduces activity). Note: Solutions need to be made up fresh daily to maintain activity. |
| Hydrogen Peroxide | Rescue® | Remove organic matter prior to disinfection (organic matter reduces activity) |
|
Glutaraldehydes |
Glutaraldehydes |
Remove organic matter prior to disinfection (organic matter reduces activity). |
|
Chlorhexidine |
Nolvasan® , Hibiclens® |
Remove organic matter prior to disinfection (organic matter reduces activity). |
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia [48] for details of anesthetic agents, procedures, and monitoring requirements
- Wash gross debris from surgical site of frog's skin with saline
- Cover the frog, around the incision site, with a drape soaked in the anesthetic water to maintain anesthesia and skin moisture
- This DOES NOT provide a sterile barrier but will keep mucus off the instruments and suture material
- Dry drapes will stick to the mucus layer and disrupt its natural protective barrier and SHOULD NOT be used
Aseptic Surgical Technique (suggested best practice)
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Unwrap sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging or instruments as this will compromise the sterility of the instruments
- Perform a surgical scrub of the surgeon’s hands
- Put on sterile gloves without touching the exterior of the glove surface
- The interior of the sterile glove packaging may be used as a sterile field for instruments
- Dedicate instruments used to incise skin solely for that purpose
- Separate these instruments from those used to manipulate exposed tissue and organs
- Maintain sterility of gloves and instruments throughout the surgery
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Aseptic Tip Technique
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Start with clean instruments (free of gross debris) that have had their tips sterilized with a dry bead sterilizer
- Wash hands or perform surgical scrub
- Put on sterile or procedural gloves
- The interior of the sterile glove packaging may be used as a sterile field for instruments if sterile gloves are used
- Arrange the instruments so that the tips are within a sterile field and the handles are outside the sterile field
- Do not contaminate the sterile tips of the instruments during this process
- Instrument tips must be maintained within this sterile field throughout the surgery
- Utilize only the sterile tips of the instruments inside the body cavity
- Use a dry bead sterilizer to sterilize the tips of surgical instruments in between multiple surgeries.
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Wound closure
- Close incision in two layers (muscle then skin)
- Close muscle with absorbable suture
- Include the white fascia membrane over the muscle layer
- Close skin with absorbable or non-absorbable suture
- DO NOT use gut suture as it may dissolve in water prior to wound healing
- PDS or monofilament Vicryl suture can be used
- Close muscle with absorbable suture
- Utilize an interrupted suture technique when closing the skin
Anesthetic Recovery and Post-Surgical Monitoring
- Recover the animal from anesthesia according to the IACUC Guidelines on Anesthesia [48]
- Place frog in fresh non-chlorinated water with head elevated above the water line to prevent drowning until conscious
- Return frog to animal housing only once it has fully recovered (i.e., swimming and diving)
- Assess the animals at least daily (including weekends and holiday) for at least three days post-operatively or as justified in the Animal Protocol
- Monitor animals for signs of pain (Pain Recognition by Species [54]) and contact OAR Veterinary Staff if signs are observed
- Monitor incision site for the following:
- Incisional integrity (i.e. sutures intact and wound is closed)
- Incision site infection: redness, excessive swelling or discharge
- Excessively tight sutures
- Remove suture in the skin layer at 10-14 days post-surgery if non-absorbable suture was used or if absorbable suture is still present
- Consult with Office of Animal Resources Veterinary staff if problems arise
Multiple Oocyte Harvest Surgeries
- Maintain surgical records (see below) identifying how many surgeries have been performed on each frog
- Up to five survival surgeries may be performed on each individual frog
- The sixth surgery must be performed as a terminal procedure
- Perform surgery on alternating sides of the abdomen with a minimum interval of two weeks between surgeries
- A minimum of four weeks should occur between surgeries if you must harvest/implant oocytes on the same side
Record Keeping
- Record the following information in addition to the anesthesia record requirements [49] Note: the anesthesia record may be combined with the surgical record; click here for the template [55]
- Procedure
- Date, time, dose and route of each analgesia administration (if applicable)
- Date when wound closures are removed/are no longer present
- Post-operative monitoring observations, including the date and time of each observation and a brief description of the animal’s health status and incision site appearance
- Reflect the animal’s health status by commenting on animal’s behavior:
- Normal behavior
- Abnormal behavior
- Keep surgery, anesthesia and post-operative records readily accessible for review
Last Reviewed by the IACUC 12/11/2019
Training Requirements for Personnel on an Animal Protocol (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose
The purpose of this policy is to clarify what training is required for personnel to be listed and approved on an Animal Protocol. For the modules noted below, a passing grade of 80%+ must be achieved.
Further information on personnel training can be found on the IACUC website as well as a link to the training request form: https://animal.research.uiowa.edu/personnel-amendments [56]
*Training request form submission is required to receive instructions on the online modules noted below.
- Online Modules
- All personnel on a protocol must complete:
- Working with the IACUC – Refresher Course. (CITI)
Note: Working with the IACUC- Basic Course is more extensive, but will be equally accepted for completion of this course requirement.
- A specific course for each species the personnel will be interacting with. (CITI)
For example, if working with mice, complete the Working with Mice in a Research module.
- If the personnel is performing surgery (non-survival, survival, or both), they must also complete:
- Aseptic Surgery Course. (CITI)
- If the personnel is performing survival surgery, they must also take (in addition to the CITI module noted above):
- IACUC Aseptic Surgery Training. (ICON/CANVAS)
- Occupational Health & Safety Program Questionnaire
- All personnel working with vertebrate animals must complete a brief questionnaire that is submitted to University Employee Health (UEHC).
- Protocol Specific Training
- For study and lab specific techniques described in the Animal Protocol, the protocol must describe the current experience the individual has and/or how the individual will be trained in the relevant techniques to be performed.
- Needed training can occur by (examples) the PI, Co-investigators, experienced personnel listed as technical personnel, and/or IACUC or OAR staff.
Optional hands-on training courses are offered. See: https://animal.research.uiowa.edu/mouse-technique-training-lab [57]
Items 1-3 are the minimum requirements to be approved as personnel on the Animal Protocol. However, it is pertinent to note that additional training items do periodically apply.
- Additional Training items
- IACUC Annual Refresher Training through ICON/Canvas
- Each year a notice will be sent to all personnel approved on an Animal Protocol to complete a course outlining current topics of relevance to the animal care and use program that all research personnel should be knowledgeable of.
- If events occur for which an individual is found to be inadequately trained, requirements for necessary retraining will be determined and communicated by the IACUC Office on a case-by-case basis.
- Separate from the IACUC required training, individuals seeking approval to enter animal facilities must complete OAR required items.
See: https://animal.research.uiowa.edu/animal-facility-access [58]
Note: The University of Iowa transitioned from an ICON animal use course to CITI in 2016. Personnel who previously only completed the ICON course, departed the University of Iowa, and are returning to University of Iowa (either for employment or visiting) will be required to complete the current CITI modules in order to be re-approved on an Animal Protocol. If certificates of completion of the CITI courses can be provided, considerations will be made to accept these completions in lieu of recertification.
Last Reviewed by the IACUC 4/10/2024
Transportation of Animals (Policy)
Animal Transportation Policy
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: The purpose of this policy is to describe approved methods for transportation of animals between the University of Iowa housing facilities and laboratories. All animal transportation must be performed by Office of Animal Resources (OAR) personnel or the laboratory personnel listed on the IACUC approved Animal Protocol. Animals cannot be transported into patient areas without prior approval.
NEVER LEAVE THE ANIMALS ALONE/UNATTENDED outside of a secured animal housing facility or laboratory
Transportation of animals between the University of Iowa Animal Facilities and other Research Facilities
- Prior approval for the transportation and transfer of animals between both units must be obtained
- Please contact OAR (319) 335-7985 for details on how to arrange movement of your animals
Transportation of animals or animal caging outside of an OAR animal housing facility
-
Rodents & Small Non-rodents
- A maximum of two cages/carriers per person may be hand carried; Larger numbers of cages or carriers should be transported using a cart
- Rodent cages may not be stacked more than 2 high
- Non-rodent carriers may not be stacked
- Check doors of carriers to ensure they are secured by pulling on the door before exiting the housing room
- To exit the animal housing facility, the cages/carriers must be:
- Covered using a clean breathable drape or similar material, OR
- Placed in a breathable, opaque, ventilated container
- A maximum of two cages/carriers per person may be hand carried; Larger numbers of cages or carriers should be transported using a cart
- Movement outside of buildings may only be performed if no interior route is available
- If transporting outside of buildings, cages/carriers must be:
- Cage tops must be secured via an OAR approved method
- Ex: lid secured with binder clips or rubber bands
- Cages must be placed in a breathable, opaque, ventilated, sanitizable secondary enclosure; such as a plastic tub with a lid and air holes
- Cage tops must be secured via an OAR approved method
- Carrier must be secured with a sturdy secondary latching mechanism
- If transporting outside of buildings, cages/carriers must be:
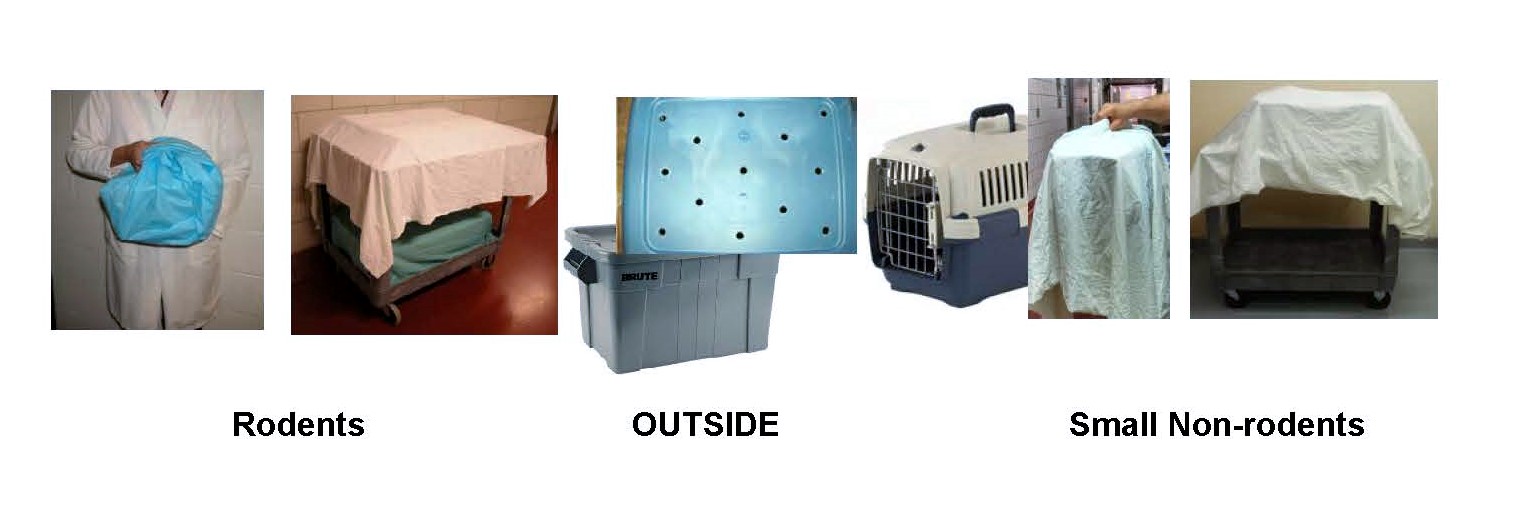
-
Larger Animals
- Animals should be transported inside a wheeled transport
- At least two people per transport must be present when loading, moving and unloading the transport
- Wheeled transport must be secured with at least two sturdy latching mechanisms on each opening (examples: Carabineer clips or electrical ties (with scissors to remove))
- Doors should be checked to ensure they are properly locked by pulling the door
- Wheeled transports must be covered using a clean breathable drape or similar material
- Wheeled transport must be sanitized between animals
- Movement outside buildings may only be performed if no interior route is available and must utilize an IACUC inspected motor vehicle that is temperature controlled

Transportation of animals between two different University of Iowa Campus locations (East Campus, West Campus and UI Research Park)
- Movement between two campus locations must be requested through OAR by submitting a transfer slips/requests completed to document the animal movement
- Contact OAR (319) 335-7985 for details on how to arrange movement of your animals
- A fee may be incurred if the animal movement is outside of the scheduled route
- Contact OAR (319) 335-7985 for details on how to arrange movement of your animals
- An IACUC approved and temperature controlled motor vehicle must be used for transportation
- Use of an OAR motor vehicle must be reserved in advance
- Use of a non-OAR motor vehicle must be approved by the IACUC
- Cages, carriers and wheeled transports must be secured while in the motor vehicle
- Wheeled transports may require strapping
- Rodent cages must not be stacked more than two cages high
- Where available, the motor vehicle must be completely enclosed in the loading dock with the loading dock door closed before the cargo door of the motor vehicle is opened
Transportation of animals between the University of Iowa Animal Facilities and the Veterans Administration Animal Research Facility in Iowa City
- Prior approval for the transportation and transfer of animals between both units must be obtained and approved in the Animal Protocols (ACORP or University of Iowa AP)
- When transporting up to two cages of animals between the VA and UI lab space the lab will assure that instructions as listed on Transportation of animals or animal caging outside of an OAR animal housing facility above are followed
- Cages are carried by hand; use of carts is not allowed
- No more than two cages are transported in this fashion
- When transporting more than two cages of animals between the VA and UI lab/procedural space the lab will assure that instructions as listed on Transportation of animals between two different University of Iowa Campus locations above are followed
- OAR must be contacted to schedule the transportation of animals that require the use of an animal receiving loading dock
- OAR must be contacted to schedule the transportation of animals that require the use of an animal receiving loading dock
Last reviewed by the IACUC 4/9/2025
Xenopus (Policy)
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Purpose: The purpose of this document is to describe when Xenopus species are covered by the Public Health Service Policy (PHS) on Humane Care and Use of Laboratory Animals. In addition this document will describe when it is required for Xenopus to be housed in OAR facilities. This document will also describe husbandry standards for Xenopus species housed in satellite housing areas. This document is intended for use by researchers and research staff at the University of Iowa.
Requirement for Submission of an Animal Protocol1
- Live embryonated eggs are only applicable to the PHS Policy once they hatch. Any Xenopus sp. used beyond Nieuwkoop and Faber stage (NF) Stage 36 (when hatching begins) requires an IACUC approved Animal Protocol.
- Adult males and females producing/laying the eggs are covered by the Policy and thus require an IACUC approved Animal Protocol
Housing Location
- Housing of Xenopus beyond Nieuwkoop and Faber (NF) stage 36 outside of OAR vivaria must be described in an Animal Protocol and approved by the IACUC
- Larval forms of frogs may be housed in the lab with prior IACUC approval until they are mature (i.e. hormone responsive)
- Housing within OAR vivaria is required once frogs are mature
- Xenopus laevis (length from nose to cloaca):
- 5-9 cm (1.9-3.5 inches) = males mature (hormone responsive);
- 6.5-9+ cm (2.5-3.5+ inches) = females mature (hormone responsive)
- Xenopus tropicalis:
- > 6 months = adult (hormone responsive)
- Xenopus laevis (length from nose to cloaca):
Monitoring of Environmental Conditions (room/enclosure temperature) *
- Housing temperature must be monitored continuously and logged at least once a day (see record keeping)
- A remote access environmental monitoring system must be used
- Alert capabilities must be enabled and programmed to contact key lab personnel when temperature is out of range
- Examples of environmental monitoring devices include:
Record Keeping
- Husbandry log/records are required starting at NF stage 36-45 and must be kept in the housing room
- Animals must be fed at least daily or more often as required when appropriate for the NF stage (example: most tadpoles may start to be fed twice a day at NF stage 55)
- The following must be documented:
- For Xenopus NF stage 36-45
- Daily recordings
- Room/enclosure temperature
- Observation of tadpole health
- When the activity occurs
- Water changes*
- Feeding *
- Records must indicate the age of Xenopus in any given container
- Daily recordings
- For Xenopus NF stage 45 until hormonally mature
- Daily recordings
- Room/enclosure temperature
- Feeding
- Observation of tadpole/froglet health
- When activity occurs
- Water changes*
- Records must indicate the age of Xenopus in any given container
- Daily recordings
* If unfamiliar with Xenopus husbandry practices, please contact an OAR veterinarian for guidance
References:
- https://olaw.nih.gov/faqs#/guidance/faqs [61]
- https://www.enasco.com/c/Education-Supplies/Xenopus-Frogs [62]
Last Reviewed by the IACUC 12/13/2023
Zebrafish (Policy)
IACUC Policy
Policy: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Policy must be described and justified in the Animal Protocol and approved by the full IACUC at a convened monthly meeting.
Zebrafish
Purpose
The purpose of this document is to describe when zebrafish species are covered by the Public Health Service Policy (PHS) on Humane Care and Use of Laboratory Animals. In addition, this document will describe when it is required for zebrafish to be housed in OAR facilities. This document is intended for use by researchers at the University of Iowa.
Requirement for Submission of an Animal Protocol1
- Live embryos are only applicable to the PHS Policy once they hatch (larvae typically hatch from their chorion or egg shell between 3-4 days post fertilization (dpf)).
- However, the adult females laying the eggs are covered by the PHS Policy and their use requires an IACUC approved Animal Protocol
- Larval forms of fish have vertebrae and are covered by the PHS Policy; thus an IACUC approved Animal Protocol is required to cover all research procedures utilizing larval forms of fish
Housing Location
- Housing of adult zebrafish (greater than 14 dpf) outside of OAR vivaria must be described and justified in the Animal Protocol and approved by the IACUC
- Larvae up to 14 dpf can be kept in a laboratory space approved by the IACUC.
- Larvae up to 14dpf will be housed in conditioned clean water.
- No additional water quality monitoring is necessary for fish up to this age
Monitoring of Environmental Conditions (room/enclosure temperature)*
- Housing temperature must be monitored continuously and logged at least once a day (see record keeping)
- A remote access environmental monitoring system must be used
- Alert capabilities must be enabled and programmed to contact key lab personnel when temperature is out of range
- Examples of environmental monitoring devices include:
Record Keeping
- Husbandry log/records are required starting at 3-4 dpf and must be kept in the housing room
- Animals must be fed at least daily when appropriate for the dpf stage (example: most zebrafish may start to be fed at day 4 dpf)2
- The following must be documented:
- For Zebrafish 3-4 dpf through 14 dpf
- Daily recordings
- Room/enclosure temperature
- Observation of fish health
- When the activity occurs
- Water changes*
- Feeding of fish*
- feeding practices may be delayed/start later than 4 dpf based on developmental stage and presence of a yolk sac
- if this occurs, it must be documented and justified in the husbandry log
- Records must indicate the age of Zebrafish in any given container
- Daily recordings
- For Zebrafish greater than 14 dpf
- Daily recordings
- Room/enclosure temperature
- Feeding of fish
- Observation of fish health
- When activity occurs
- Water changes *
- Daily recordings
- Records must indicate the age of Zebrafish in any given container
* If unfamiliar with zebrafish husbandry practices, please contact an OAR veterinarian for guidance
References:
- Office of Laboratory Animal Welfare (OLAW) FAQ: http://grants.nih.gov/grants/olaw/faqs.htm [63]
- The Zebrafish Information Network (ZFIN): https://zfin.org/zf_info/zfbook/chapt3/3.2.html [64]
Last reviewed by the IACUC 12/13/2023