Breadcrumb
- Home
- Office of the IACUC
- Policies, Guidelines and Informational Sheets
- Anesthesia (Guideline)
Anesthesia (Guideline)
Main navigation
- IACUC Contacts
- IACUC News
- IACUC AnShare ListServ
- Animal Protocol
-
Policies, Guidelines and Informational Sheets
- Anesthesia (Guideline)
- Analgesia - Buprenorphine ER (Informational Sheet)
- Analgesia (Guideline)
- Biologic Testing - Guidance and Procedures for Rodent Biologic Testing (Informational Sheet)
- Blood Collection (Guideline)
- Breeding - Rodent Breeding Colony Management (Policy)
- Enrichment - Canine Enrichment and Exercise Program
- Enrichment - Environmental Enrichment Program
- Euthanasia (Guideline)
- Euthanasia - Confirmation of Death (Policy)
- Euthanasia by OAR Personnel (Policy)
- Genotyping - Mouse Toe Clipping (Policy)
- Genotyping - Rodent Tail Snipping for Genotyping (Policy)
- Hazardous Agent Containment (biohazards, chemical hazards, & radioactive materials)
- Humane Intervention Points (Guideline)
- Media - Social Media (Policy)
- Media - Media Security (Policy)
- New Weanling Procedure for Labs (Guideline)
- Outbreak - OAR Pathogen Outbreak Control Plan
- Pain Recognition in Laboratory Animals (Informational Sheet)
- Principal Investigator Eligibility for Animal Protocols (Policy)
- Satellite Housing Expectactions (Policy)
- Social Housing of Species (Policy)
- Sterilization - Accepted Methods & Monitoring (IACUC Guideline)
- Substance Administration - Recommended Volumes (Informational Sheet)
- Substance Administration - Use of Drugs and Chemicals in Laboratory Animals (Guideline)
- Surgery - Non-Survival Surgery (Guideline)
- Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
- Surgery - Rodent Blood Loss (Informational Sheet)
- Surgery - USDA Covered Species Survival Surgery (Guideline)
- Surgery - Xenopus Oocyte Harvest (Guideline)
- Training Requirements for Personnel on an Animal Protocol (Policy)
- Transportation of Animals (Policy)
- Xenopus (Policy)
- Zebrafish (Policy)
- Personnel Training
- Educational Materials
- NIH Grant Information
- Occupational Hazards Associated with the Care and Use of Laboratory Animals
- DEA Information
- New Faculty/Recruitment
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to provide guidance on commonly used inhaled and injectable anesthetic agents for use in animal research at the University of Iowa. All anesthetic agents used in animals must be listed on an approved Animal Protocol. All anesthetic procedures must be performed by appropriately trained personnel.
Recordkeeping for Anesthesia/Sedation
- Applies to all survival and non-survival procedures performed under anesthesia
- Mice and Rats (as well as fish, amphibians, reptiles, and birds)
- Brief procedures using inhalant/absorbed anesthetics
- No anesthesia record is required if anesthesia is less than 5 minutes.
- Use of eye lubrication is not required for anesthesia procedures lasting less than 5 minutes but is strongly recommended.
- Brief procedures are those that cause no more than momentary pain (e.g. blood collection, tail vein injection, tail snip for genotyping or intranasal inoculations, fin snip on zebrafish)
- No anesthesia record is required if anesthesia is less than 5 minutes.
- For all other procedures not described above
- An anesthesia record is required.
- Eye lubrication is required.
- Animals should be monitored from anesthetic agent administration until full recovery if survival or euthanasia if non-survival.
- Document the following:
- Date
- Principal Investigator
- Animal Protocol number
- Animal ID
- Species
- Weight
- Procedure
- Agent(s) used, dosage, route of administration
- Time of anesthetic agent administration
- Time of recovery from anesthesia or time of euthanasia
- Brief procedures using inhalant/absorbed anesthetics
- USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
- Any anesthetic event
- An individual record for each animal is required
- Document the following:
- Date
- Principal Investigator
- Animal Protocol number
- Animal ID
- Species
- Weight
- Procedure
- Agent(s) used, dosage, route of administration
- Time of anesthetic agent administration
- Time of recovery from anesthesia or time of euthanasia
- Monitoring (every 15 minutes from anesthetic agent administration until recovery or euthanasia)
- Any anesthetic event
- Please see the surgery guidelines for surgical record information
- Click here for template anesthesia records
Supportive Care of Animals During Anesthesia
- Apply ophthalmic ointment to both eyes to prevent desiccation for any anesthesia longer than 5 minutes.
- Rodents can quickly become hypothermic under anesthesia that is longer than 5 minutes and heat support is required. Maintain normal body temperature using an allowed heating support option (below).
- Provide fluids (e.g., IV, IP, SQ) to animals during prolonged anesthesia to maintain adequate hydration as described in the approved Animal Protocol
Allowed Heating Support Options:
- Warm circulating water blanket, thermal pads, and/or warmed IV fluids.
- Electric heating pads must have temperature setting capability and digital readout. Recommended heating pad options include but are not limited to the following:
- Kent Scientific Far Infrared Warming
- Parkland Scientific Lab Animal Warm Water Blankets and Circulators
- Conduct Science Heating Pads
- Braintree scientific Delta Phase Isothermal Pads and Insulators
- Please contact OAR Veterinary Care Staff to review alternative heating pad options.
Prohibited Heating Support Options:
- DO NOT use an “over the counter” low/medium high setting electric heating pad as these are prone to overheating
- Common Brands: Sunbeam, Pure Relief, Geniani, etc.
Monitoring and Assessment of Anesthesia (while procedure is being performed)
- USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
- Monitor heart rate, respiratory rate, and temperature
- Document these parameters at least every 15 minutes starting at the time of anesthetic agent administration until the animal is recovered or euthanized
- For rodent species, qualitative monitoring for normal cardiovascular and respiratory function may be sufficient, as it is difficult to assess these parameters quantitatively.
- For all other species, quantitative monitoring (numerical heart and respiratory rates) is expected.
- If using a ventilator, note ventilation rate and tidal volume in the records
- Monitor hemodynamic parameters to assure adequate gas exchange
- Mucous membranes should be pink and moist
- Capillary refill time should be less than 2 seconds
- Monitoring for at least one indicator of deep pain recognition (pedal reflex, pinna reflex, etc.) should be performed regularly to ensure adequate anesthesia
- Adjust the depth of anesthesia as dictated by changes in the monitored parameters to ensure continued surgical plane of anesthesia
- Monitor heart rate, respiratory rate, and temperature
- Mice and Rats
- Monitor respiratory rate and effort, color of mucous membranes, and reflected eye color (in albino animals) at regular intervals (no longer than 15-minute intervals)
- Assess level of anesthesia by pedal reflex (firm toe pinch) and adjust anesthetic delivery as appropriate to maintain surgical plane
Anesthetic Recovery
- Large Animals
- Food and water bowls must be removed from the recovery cage
- Monitor each animal continuously until holding sternal position:
- Maintaining sternal recumbency (lying upright on chest)
- Heart and respiratory rate
- Body temperature
- A circulating warm water heating pad is recommended
- Hydration as assessed by skin turgor or mucous membrane “tackiness”
- Monitor each animal at least every 15 minutes until ambulatory (walking normally)
- Deflate and remove the intubation tube once animal can swallow
- Do not leave animal unattended while intubation tube is in place
- Occasionally reposition recumbent animals to promote a quicker recovery
- Animals should not remain with the same side down for more than four (4) hours
- Lower animal’s head slightly below chest level to prevent aspiration if vomiting occurs
- Mice and Rats
- Place rodent in warm, clean, dry, quiet environment away from other animals
- Cover or replace bedding material with toweling material
- Bedding can stick to eyes or be inhaled while animals are recovering from anesthesia
- Provide warmth during recovery (required if anesthesia is greater than 5 minutes). Maintain normal body temperature using:
- An allowed heating support option (see above). *Note, when using an allowed heating pad option during anesthesia recovery, place cages so that approximately 50% of the cage is on the allowed heating pad option. This allows the animal to move away from the heat as it recovers from anesthesia.
- Incandescent lamp (50-75 watt) 12-14 inches away from rodent
- Position lamp so that rodent can escape the light sources if desired
- Attentive monitoring must be performed to prevent overheating of rodent
- Use of a temperature-controlled cage/incubator
- If listed in approved Animal Protocol, warm sterile saline can be administered to replace body fluids lost during surgery
- All animals must be continuously visually monitored (i.e. present in the room with ability to see the animal) until maintaining upright posture and walking normally about the cage before completion of monitoring and return to the animal housing room. Physiological parameters must be monitored at least every 15 minutes as noted above.
Anesthetic Agents
The following is a list of commonly used anesthetic agents. This list is not inclusive; other anesthetic agents may be listed and used in an Animal Protocol.
Other accepted resources for appropriate analgesics include the following formularies:
- Plumb’s Veterinary Drug Handbook (Plumb)
- Anesthesia and Analgesia in Laboratory Animals (ACLAM)
- Formulary for Laboratory Animals (Hawk)
- Exotic Animal Formulary (Carpenter & Marion)
- Handbook of Veterinary Anesthesia (Muir & Hubbell)
- Swine in the Laboratory (Swindle)
Appropriate anesthetic drugs and dosage(s) should be determined in consultation with an OAR or IACUC veterinarian.
Inhaled Anesthetic Agents
ISOFLURANE/SEVOFLURANE - VAPORIZER
- Isoflurane/sevoflurane must be administered with a properly calibrated vaporizer when used as an anesthetic agent for surgery
- Anesthetic gases must be scavenged properly
- Direct exhaust
- Activated charcoal canister (e.g. F/Air canister)
- Canister must be discarded when it reaches the maximum weight or use time as defined by the manufacturer, whichever comes first
- “Use time” is the time anesthesia gas is flowing / in use
- Should be weighed before each use
- Canister must be discarded when it reaches the maximum weight or use time as defined by the manufacturer, whichever comes first
Weight and time records must be maintained
- Vaporizers must be calibrated at least yearly
- Calibration records for each vaporizer must be maintained
ISOFLURANE – DROP JAR METHOD
- NOT for sevoflurane use; the concentration of sevoflurane cannot be accurately controlled with the drop jar method.
- Ensure that use is approved in the Animal Protocol.
- Isoflurane can be administered in an anesthetic drop jar for a single, brief procedure
- Cannot be used for any surgical procedures (includes non-survival and survival surgeries) or non-brief methods of euthanasia (e.g. perfusion)
- Anesthetic gases must be scavenged properly
- Fume hood
- Hard-ducted biosafety cabinet
- Animals must be clearly visible within the container.
- Animals must not come into direct contact with liquid isoflurane
- Anesthetize one animal at a time.
- Drop jar must be cleaned (i.e. removal of urine/feces) between animals with appropriate disinfectants.
- 70% EtOH is a good cleaner but is a weak disinfectant.
- It is required that labs use a strong disinfectant for cleaning animal use surfaces. (see examples of appropriate hard surface disinfectants here.)
- Use of this method with a 50 mL conical tube is prohibited.
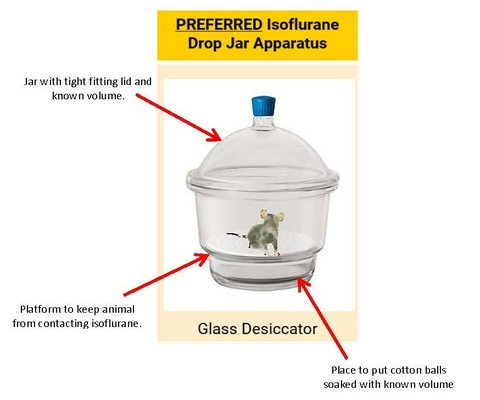
Drop jar dosing for Isoflurane: Internal Volume of Chamber (L) and isoflurane liquid required (mL)
| Isoflurane Concentration achieved | 1L | 2L | 3L | 4L | 5L |
|---|---|---|---|---|---|
| 1% | 0.05mL | 0.10 mL | 0.15 mL | 0.20 mL | 0.26 mL |
| 2% | 0.10 mL | 0.20 mL | 0.31 mL | 0.41 mL | 0.51 mL |
| 3% | 0.15 mL | 0.31 mL | 0.46 mL | 0.61 mL | 0.77 mL |
| 4% | 0.20 mL | 0.41 mL | 0.61 mL | 0.82 mL | 1.02 mL |
| 5% | 0.26 mL | 0.51 mL | 0.77 mL | 1.02 mL | 1.28 mL |
75% CO2 / 25% O2
- 75% CO2 / 25% O2 can be used for single, brief procedures
- Cannot be used for any surgical procedures (includes non-survival and survival surgeries).
- Available pre-mixed from vendors
- Use precaution; animals can be easily overdosed
- Not intended for euthanasia
- Contact a member of the Office of Animal Resources veterinary staff if you are unfamiliar with the proper use of CO2/O2
Absorbed Anesthetic Agents
- Tricaine Methanesulfonate (MS-222)
- Frogs:
- 0.05-0.2% (500-2000mg/L) solution
- Solution must be buffered with sodium bicarbonate to a pH of 7.0-7.5
- Immerse frog in solution for 10-20 minutes
- Level of anesthesia is judged by loss of righting reflexes, loss of gulping reflex and loss of withdrawal response to toe pinch
- Fish:
- 0.0025-0.01% (25-100mg/L) solution
- Solution must be buffered with sodium bicarbonate to a pH of 7.0-7.5
- Immerse fish in solution until appropriate anesthetic depth is observed
- Level of anesthesia is judged by loss of equilibrium, loss of response to noxious stimuli (pinching base of tail), rate of opercular movement and gill color
- Storage
- Tricaine (liquid solution) can be stored at room temperature for 3-5 days if protected from exposure to light.
- Tricaine (liquid solution) can be stored at 4°C (i.e. – the refrigerator) for 1 month if stored if protected from light.
- Tricaine (liquid solution) can be stored at -20°C (i.e. – the freezer) for 1 year if protected from the light.
- Tricaine (powder) can be stored at room temperature for up to 5 years if stored in a dark container.
- Frogs:
Injectable Anesthetic Agents
COMMONLY USED INJECTABLE ANESTHETIC AGENTS
MOUSE
Agent | Dosage | Duration of anesthesia |
|---|---|---|
Ketamine/xylazine* | ketamine 80-100 mg/kg IP xylazine 10-12.5 mg/kg IP | 20-30 minutes |
Ketamine/xylazine cocktail* | KX mouse cocktail 0.1mL/20g mouse wt. IP Contains: 87.5 mg/kg Ketamine 12.5 mg/kg Xylazine | 20-30 minutes |
Ketamine/xylazine/acepromazine | ketamine 60-100 mg/kg IP xylazine 10-15 mg/kg IP acepromazine 2-5 mg/kg IP | 60-90 minutes |
Pentobarbital | 50 mg/kg IP | 20-40 minutes |
Avertinǂ See warning below | 240 mg/kg IP | 30 minutes |
*Ketamine/xylazine without combination with an analgesic agent (opioid or NSAID) may be insufficient to produce a surgical plane of anesthesia. Administration of appropriate analgesic agents prior to surgery and/or addition of acepromazine will augment the anesthetic effect of ketamine/xylazine.
** Preparation instructions for the ketamine/xylazine cocktail may be found below.
ǂ WARNING: NIH and European guidelines discourage the use of Avertin. Preparation and storage requirements for Avertin may be found below.
* GUIDELINES - PREPARATION OF KETAMINE/XYLAZINE COCKTAIL FOR MICE
- Use of a sterile injection vial is required (e.g. redtop blood collection tube; commercial injection vial)
- Mixing instructions:
- Verify the concentration of your drugs prior to mixing
- For a 10mL vial using ketamine 100 mg/mL and xylazine 100 mg/mL add:
- 1.75mL ketamine (100 mg/mL)
- 0.25 mL xylazine (100 mg/mL)
- 8 mL saline or sterile water for injection
- Use of the following template for a label is recommended:
- Mouse Anesthetic Mix: Ketamine/Xylazine
- Dosage: 0.1 ml/ 20gm IP
- Delivers: 87.5 mg/kg Ketamine/12.5 mg/kg Xylazine
- Concentration: 17.5 mg/mL Ketamine/2.5 mg/mL Xylazine
- Expires: ____________
-
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
- E.g.: Diluted on 8/13/21, ketamine expires 12/10/2022, xylazine expires 10/10/21 and sterile water for injection expires 1/12/2023; the expiration date for the cocktail is 10/10/21
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
RAT
Agent | Dosage | Duration of anesthesia |
|---|---|---|
Ketamine/xylazine | ketamine 40-100 mg/kg IP xylazine 5-13 mg/kg IP | 60-80 minutes |
Ketamine/xylazine cocktail*
| KX rat cocktail 0.1 mL/100g rat wt. IP Contains: 91 mg/kg Ketamine 9.1 mg/kg Xylazine | 60-80 minutes |
Ketamine/xylazine/acepromazine | ketamine 20-50 mg/kg IP xylazine 2-10 mg/kg IP acepromazine 0.5-1.5 mg/kg IP | 60-120 minutes |
Pentobarbital | 30-50 mg/kg IP | 90-120 minutes |
*Ketamine/xylazine without combination with an analgesic agent (opioid or NSAID) may be insufficient to produce a surgical plane of anesthesia. Administration of appropriate analgesic agents prior to surgery and/or addition of acepromazine will augment the anesthetic effect of ketamine/xylazine.
** Preparation instructions for the ketamine/xylazine cocktail may be found below.
GUIDELINES - PREPARATION OF KETAMINE/XYLAZINE COCKTAIL FOR RATS
- Use of a sterile injection vial is required (e.g. redtop blood collection tube; commercial injection vial)
- Mixing instructions:
- Verify the concentration of your drugs prior to mixing
- For a 10mL vial using ketamine 100 mg/mL and xylazine 100 mg/mL add:
- 10 mL ketamine (100 mg/mL)
- 1 mL xylazine (100 mg/mL)
- Use of the following template for a label is recommended:
- Rat Anesthetic Mix: Ketamine/Xylazine
- Dosage: 0.1 ml/ 100gm IP
- Delivers: 91 mg/kg Ketamine, 9.1 mg/kg Xylazine
- Concentration: 91 mg/mL Ketamine, 9.1 mg/mL Xylazine
- Expires: ____________
-
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
- E.g.: Diluted on 8/13/21, ketamine expires 12/10/2022, xylazine expires 10/10/21 and sterile water for interjection expires 1/12/2023, the expiration date for the cocktail is 10/10/21
- The expiration date for the cocktail is determined by either six months from the mixing date, or whichever of the components expires first (if less than 6 months)
RABBIT
| Agent | Dosage |
|---|---|
| Ketamine/xylazine | ketamine 22-50 mg/kg IM xylazine 2.5-10 mg/kg IM |
| Pentobarbital | 20-60 mg/kg IV |
PIG
| Agent | Dosage |
|---|---|
| ketamine/xylazine | ketamine 20 mg/kg IM xylazine 2 mg/kg IM |
| Telazol/ketamine | telazol 4.4 mg/kg ketamine 2.2 mg/kg |
| Pentobarbital | 20-40 mg/kg IV |
SHEEP
| Agent | Dosage |
|---|---|
| Ketamine/xylazine | 5-15 mg/kg IM ketamine 0.05-0.2 mg/kg IM xylazine |
| Thiopental | 10-16 mg/kg IV |
FERRET
| Agent | Dosage |
|---|---|
| Ketamine/xylazine | 10-25 mg/kg IM ketamine 0.25-0.5 mg/kg IM xylazine |
Other species or anesthetic agents:
Please contact a University of Iowa clinical veterinarian(link sends e-mail) for consultation
GUIDELINES FOR PREPARATION AND STORAGE OF AVERTIN (TRIBROMOETHANOL)
- Avertin is a quick-acting, non-pharmaceutical grade* anesthetic that is used for short duration surgical procedures in mice.
- NOTE: Per the Guide for the Care and Use of Laboratory Animals 8th edition, the use of non-pharmaceutical grade chemicals or substances needs to be described and scientifically justified in the Animal Protocol.
- Precautions:
- Do not administer Avertin if you have a/an:
- Non-sterile solutions
- Outdated solutions
- More concentrated solutions
- Higher dosages than recommended
- Avertin should only be administered one time (no redosing) due to resultant gastrointestinal irritation
- Do not administer Avertin if you have a/an:
- Disadvantages of the use of Avertin:
- Tissue irritation, especially at high dosages, high concentrations or repeated doses
- Degrades in the presence of heat or light to produce toxic byproducts which can be both nephrotoxic and hepatotoxic
- Can cause intestinal ileus several weeks after injection
- Unpredictable effects in mice under 16 days of age or in mice with altered carbohydrate metabolism (e.g., mouse strains used as diabetes or obesity models)
- Some European journals are rejecting research manuscripts when Avertin is used as an anesthetic
- Ingredients:
- 2.5 grams 2,2,2 Tribromoethanol
- 5 ml 2-methy-2-butanol (amylene hydrate, tertiary amyl alcohol)
- 200 ml distilled water - neutral pH (sterile)
- Preparation (12.5 mg/ml solution):
- Dissolve 2.5 grams Tribromoethanol in 5 ml amylene hydrate.
- Heat dissolved solution to 40°C while stirring vigorously.
- Do not exceed 40°C.
- Add distilled water, stirring continuously, up to a final volume of 200 ml.
- Filter sterilize through a Millipore filter (0.5 micron)
- Storage:
- Filter final solution into red-cap blood collection tubes or amber (brown) colored sterile glass containers
- Solution container must be wrapped in aluminum foil to protect solution from light
- Solution container must be labeled with contents and date of preparation
- Store in refrigerator or freezer
- Expiration has occurred if any one of the following conditions are met:
- Two week expiration date if stored in refrigerator
- One year expiration date if stored in freezer
- Crystallization of solution
- Solution has turned yellow in color
Last Reviewed by the IACUC 2/11/2026