Breadcrumb
- Home
- Office of the IACUC
- Policies, Guidelines and Informational Sheets
- Blood Collection (Guideline)
Blood Collection (Guideline)
Main navigation
- IACUC Contacts
- IACUC News
- IACUC AnShare ListServ
- Animal Protocol
-
Policies, Guidelines and Informational Sheets
- Anesthesia (Guideline)
- Analgesia - Buprenorphine ER (Informational Sheet)
- Analgesia (Guideline)
- Biologic Testing - Guidance and Procedures for Rodent Biologic Testing (Informational Sheet)
- Blood Collection (Guideline)
- Breeding - Rodent Breeding Colony Management (Policy)
- Enrichment - Canine Enrichment and Exercise Program
- Enrichment - Environmental Enrichment Program
- Euthanasia (Guideline)
- Euthanasia - Confirmation of Death (Policy)
- Euthanasia by OAR Personnel (Policy)
- Genotyping - Mouse Toe Clipping (Policy)
- Genotyping - Rodent Tail Snipping for Genotyping (Policy)
- Hazardous Agent Containment (biohazards, chemical hazards, & radioactive materials)
- Humane Intervention Points (Guideline)
- Media - Social Media (Policy)
- Media - Media Security (Policy)
- New Weanling Procedure for Labs (Guideline)
- Outbreak - OAR Pathogen Outbreak Control Plan
- Pain Recognition in Laboratory Animals (Informational Sheet)
- Principal Investigator Eligibility for Animal Protocols (Policy)
- Satellite Housing Expectactions (Policy)
- Social Housing of Species (Policy)
- Sterilization - Accepted Methods & Monitoring (IACUC Guideline)
- Substance Administration - Recommended Volumes (Informational Sheet)
- Substance Administration - Use of Drugs and Chemicals in Laboratory Animals (Guideline)
- Surgery - Non-Survival Surgery (Guideline)
- Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
- Surgery - Rodent Blood Loss (Informational Sheet)
- Surgery - USDA Covered Species Survival Surgery (Guideline)
- Surgery - Xenopus Oocyte Harvest (Guideline)
- Training Requirements for Personnel on an Animal Protocol (Policy)
- Transportation of Animals (Policy)
- Xenopus (Policy)
- Zebrafish (Policy)
- Personnel Training
- Educational Materials
- NIH Grant Information
- Occupational Hazards Associated with the Care and Use of Laboratory Animals
- DEA Information
- New Faculty/Recruitment
Guidelines:The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose:
This document provides direction and guidance on appropriate blood collection methods and volumes for animals used in research at the University of Iowa. These guidelines are intended for use by qualified personnel performing blood collection as described on an IACUC-approved Animal Protocol.
There are several factors to consider when determining the appropriate blood collection volume and technique. These include:
- The species to be sampled
- The size of the animal to be sampled
- The age and health of the animal to be sampled
- The minimum volume required for analysis
- The frequency of sampling necessary
- The training and experience of the personnel performing the collection
- The suitability of sedation and/or anesthesia
The sample volume selected should always be the minimum volume of blood which satisfies experimental needs. Appropriate restraint (physical or chemical) should be employed to minimize risk of injury to the animal and personnel.
Guidelines for calculation of collection volume:
- The maximum permitted blood volume includes blood lost during collection.
- As a general rule, 20 drops = 1 mL (i.e. 5 drops = 250 uL)
Maximal blood collection limits are as follows:
- No more than 1% of the animal’s body weight in one collection or over a 24 hour period
- For example: 25g mouse x 1% = 0.25mL or 250uL maximum blood removal
- No more than 1.5% of the animal’s body weight in two weeks (14 days)
- For example: 200g rat x 1.5% = 3.0mL maximum over 14 days
Frequent Rodent Calculations
Mouse
| Weight | Maximum blood loss at one time/ in 24 hours | Maximum blood loss over 14 days |
|---|---|---|
| 20 g | 200 uL | 300 uL |
| 25 g | 250 uL | 375 uL |
| 30 g | 300 uL | 450 uL |
Rat
| Weight | Maximum blood loss at one time/ in 24 hours | Maximum blood loss over 14 days |
|---|---|---|
| 200 g | 2.0 mL | 3 mL |
| 250 g | 2.5 mL | 3.75 mL |
| 300 g | 3.0 mL | 4.5 mL |
Common Blood Collection Routes By Species
Mouse
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Submandibular vein | ||
| Tail vein (see below) | ||
| Saphenous vein | ||
| Retro-orbital sinus (see below) | Yes | |
| Cardiac (non-survival) | Yes |
Rat
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Tail vein (see below) | ||
| Saphenous vein | ||
| Jugular vein | Yes | |
| Retro-orbital plexus (see below) | Yes | |
| Sublingual vein | Yes | |
| Cardiac (non-survival) | Yes |
Ferret
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Cephalic vein | ||
| Saphenous vein | ||
| Jugular vein | Yes | |
| Cranial vena cava | Yes |
Rabbit
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Marginal ear vein | ||
| Central auricular artery | ||
| Saphenous vein | Yes | |
| Jugular vein | Yes | |
| Cardiac (non-survival) | Yes |
Hamsters
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Saphenous vein | ||
| Cephalic vein | ||
| Jugular vein | Yes | |
| Cranial vena cava | Yes | |
| Cardiac (non-survival) | Yes |
Guinea Pigs
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Ear vein (droplet) | ||
| Saphenous vein | ||
| Cranial vena cava | Yes | |
| Cardiac (non-survival) | Yes |
Gerbils
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Lateral saphenous vein | ||
| Cranial vena cava | Yes | |
| Cardiac (non-survival) | Yes |
Xenopus
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Dorsal tarsal vein | Yes | |
| Cardiac (survival) | Yes | |
| Cardiac (non-survival) (also tadpoles) | Yes |
Pigeon
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Brachial wing vein | Yes |
Dog or Cat
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Cephalic vein | ||
| Saphenous vein | ||
| Jugular vein | ||
| Cardiac (non-survival) | Yes |
Pigs
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Ear vein | Yes | |
| Cranial vena cava | Yes | |
| Jugular vein | Yes | |
| Cardiac (non-survival) | Yes |
Ruminants
| Common Blood Collection Route(s) | Sedation Recommended | Anesthesia Required |
|---|---|---|
| Jugular vein | ||
| Lateral saphenous vein | ||
| Tail vein | ||
| Ear vein |
Restraint and anesthesia for blood draws:
Restraint methods and anesthesia used to collect blood on research animals must be described and approved in the animal protocol. Examples of restraint devices include rodent restraint tubes, surgical towel or decapicones.
Hemostasis:
Assuring that blood flow has stopped (hemostasis) is of upmost importance after collecting a blood sample. To achieve hemostasis, place gentle pressure over the site of blood collection to stop the bleeding. A gloved hand and a piece of gauze are commonly used. Best practice involves re-inspecting animals approximately 5 minutes after return to their cage to assure blood flow has stopped.
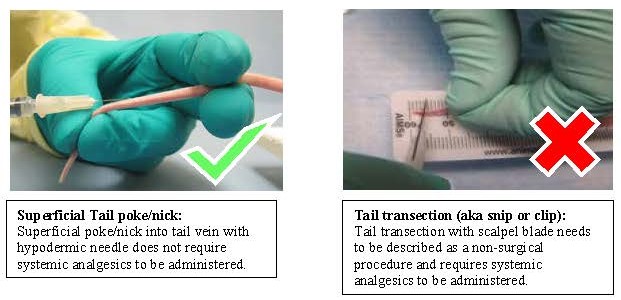
Tail vein collection definitions:
Tail vein collection is defined as use of a hypodermic needle or lancet to access the tail vein along the body of the tail.
Tail transection (also referred to as tail snip or clip) is NOT considered a routine method of blood collection. In mice only, it may be performed when described as a non-surgical procedure with associated monitoring and pain management where appropriate.
- Tail transection for blood collection of mice greater than 24 days of age:
- Use of a systemic analgesic given prior to tail transection is required.
- systemic analgesic examples: carprofen, meloxicam, buprenorphine, etc
- Analgesics such as lidocaine or bupivacaine are considered local analgesics and when administered alone, do not provide optimal pain management for this procedure.
- Tail transection for blood collection at this age is potentially painful and should be avoided if possible.
- Use of a systemic analgesic given prior to tail transection is required.
- More than one sample over the life of the animal:
- Limit of two collections, no more than a total of 2-5mm of the distal tail removed over all collections (including tail snipping for genotyping purposes)
- Use of a systemic analgesic given prior to tail transection is required.
Techniques for tail vein dilation:
The following techniques may be used to increase blood flow on the tail vein of a mouse or a rat:
1) Use of a heating lamp*
2) Submerging the tail in warm water (no warmer than 40oC/104oF) *
3) Placing rubbing alcohol over the tail
* Animals under a heat lamp must be under direct supervision and care must be exercised to prevent overheating an animal. Animals that overheat may show an increased respiratory rate, decreased movement, red extremities and avoidance of the heat lamp.
Retro-Orbital Sampling:
Retro-orbital blood collection in rodents can provide moderate to large amounts of blood when performed by well-trained personnel. However, severe injuries may occur to the animal if this procedure is not done properly, and available alternatives should be used whenever possible.
The use of retro-orbital bleeding must be described in the protocol and approved by the IACUC. Because rats have a venous plexus rather than a sinus (as in the mouse), the use of this method may result in greater tissue damage and alternative collection sites are strongly recommended.
If retro-orbital collection is necessary, the following guidelines apply:
- General anesthesia is required
- Microhematocrit tubes that hold 50-75 microliters are recommended to minimize risk of injury
- Only one eye may be sampled at any time
- If attempted collection from one eye is unsuccessful, an alternate method approved in the Animal Protocol (e.g. submandibular or saphenous route) must be used, rather than reattempting retro-orbital collection from the same or opposite eye
- Alternate between left and right eyes per session
- No more than 1 collection performed per 7 days (alternate eyes). therefore 14 days between collections in the same eye
- Exception: If repeated sampling within 8 hours is necessary and approved in the Animal Protocol, the retro-orbital sinus may be re-sampled by disrupting the blood clot (from the original collection site) without repeated damage to the sinus, provided the 24 hour maximum blood collection limits are not exceeded
- Please consult with veterinary staff for demonstration and training of proper technique to reduce risk of trauma
- Exception: If repeated sampling within 8 hours is necessary and approved in the Animal Protocol, the retro-orbital sinus may be re-sampled by disrupting the blood clot (from the original collection site) without repeated damage to the sinus, provided the 24 hour maximum blood collection limits are not exceeded
- A maximum of 3 procedures may be performed per eye (up to 6 collections total)
- If injury and/or rupture of the eye or surrounding tissues occurs due to this method, the animal must be immediately euthanized or an OAR veterinarian consulted for guidance
Application of a topical ophthalmic anesthetic during/after collection should be considered to provide post-procedural analgesia.
Last Reviewed by the IACUC 10/8/2025