Breadcrumb
- Home
- Office of the IACUC
- Policies, Guidelines and Informational Sheets
- Surgery - USDA Covered Species Survival Surgery (Guideline)
Surgery - USDA Covered Species Survival Surgery (Guideline)
Main navigation
- IACUC Contacts
- IACUC News
- IACUC AnShare ListServ
- Animal Protocol
-
Policies, Guidelines and Informational Sheets
- Anesthesia (Guideline)
- Analgesia - Buprenorphine ER (Informational Sheet)
- Analgesia (Guideline)
- Biologic Testing - Guidance and Procedures for Rodent Biologic Testing (Informational Sheet)
- Blood Collection (Guideline)
- Breeding - Rodent Breeding Colony Management (Policy)
- Enrichment - Canine Enrichment and Exercise Program
- Enrichment - Environmental Enrichment Program
- Euthanasia (Guideline)
- Euthanasia - Confirmation of Death (Policy)
- Euthanasia by OAR Personnel (Policy)
- Genotyping - Mouse Toe Clipping (Policy)
- Genotyping - Rodent Tail Snipping for Genotyping (Policy)
- Hazardous Agent Containment (biohazards, chemical hazards, & radioactive materials)
- Humane Intervention Points (Guideline)
- Media - Social Media (Policy)
- Media - Media Security (Policy)
- New Weanling Procedure for Labs (Guideline)
- Outbreak - OAR Pathogen Outbreak Control Plan
- Pain Recognition in Laboratory Animals (Informational Sheet)
- Principal Investigator Eligibility for Animal Protocols (Policy)
- Satellite Housing Expectactions (Policy)
- Social Housing of Species (Policy)
- Sterilization - Accepted Methods & Monitoring (IACUC Guideline)
- Substance Administration - Recommended Volumes (Informational Sheet)
- Substance Administration - Use of Drugs and Chemicals in Laboratory Animals (Guideline)
- Surgery - Non-Survival Surgery (Guideline)
- Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
- Surgery - Rodent Blood Loss (Informational Sheet)
- Surgery - USDA Covered Species Survival Surgery (Guideline)
- Surgery - Xenopus Oocyte Harvest (Guideline)
- Training Requirements for Personnel on an Animal Protocol (Policy)
- Transportation of Animals (Policy)
- Xenopus (Policy)
- Zebrafish (Policy)
- Personnel Training
- Educational Materials
- NIH Grant Information
- Occupational Hazards Associated with the Care and Use of Laboratory Animals
- DEA Information
- New Faculty/Recruitment
Survival Surgery of USDA Covered Species (all mammals except for mice of the genus Mus and rats of the genus Rattus)
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Non-Rodent Mammal Survival Surgery
Purpose: The purpose of these guidelines is to provide direction for personnel conducting survival surgery on research mammals (other than Mus orRattus) at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures on USDA covered species, or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Definitions
- Major Survival Surgery: Any surgical intervention that penetrates and exposes a body cavity or produces permanent impairment of physical or physiologic functions
- Minor Survival Surgery: Survival surgical intervention(s) that do(es) not expose a body cavity and/or causes little or no physical impairment
- Non-Survival Surgery: Surgical intervention(s) that are completed under a surgical plane of anesthesia from which the animal does not recover prior to euthanasia
Instrument and Equipment Sterilization
- Use only sterile instruments, surgical supplies and wound closure materials
- If a sterile instrument or sterile glove comes in contact with a non-sterile item, it is NO LONGER STERILE
- Re-sterilize or replace contaminated items before continuing aseptic procedures
- New fully sterilized supplies must be used for each animal
- Refer to sterilization methods for further information on material sterilization methods
Preoperative Considerations
- Acclimate animals to the housing facility prior to surgery (best practice; not required)
- Assess each animal’s health status prior to inducing anesthesia
- Consult with an Office of Animal Resources (OAR) veterinarian for any animal identified to have a health concern via an Orange Card and/or with any questions concerning health status
- Fast animal prior to inducing anesthesia
- Do not fast rabbits or rodents
- Prevent development of bloat in ruminants
- Utilize a 24-48 hour pre-anesthetic fasting period
- Shorten the total anesthetic period when possible
- If rumen distention occurs, immediately consult with an OAR veterinarian so that appropriate treatment may be initiated
Surgery Location
- Perform major survival surgeries in a dedicated IACUC-approved surgical suite
- Minor survival surgeries or non-survival surgeries may be performed in a dedicated suite or as listed on the Animal Protocol
Area Preparation
- Clean and disinfect all surfaces and equipment in the surgical suite with a quaternary ammonium compound or similar disinfectant using appropriate contact time.
- Establish a sterile field near the animal for placement of sterile instruments
Examples of hard surface disinfectants
| AGENT | EXAMPLES | COMMENTS |
|---|---|---|
| Quaternary Ammonium | Sani Cloth®, Roccal®, Quatricide®, Tec-Surf II® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorine | Sodium hypochlorite (Clorox® 10% solution) Chlorine dioxide (Clidox®, MB-10®) | Remove organic matter prior to disinfection (organic matter reduces activity). Note: Solutions need to be made up fresh daily to maintain activity. |
| Glutaraldehydes | Glutaraldehydes (Cetylcide®, Cide Wipes®) | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Phenolics | Lysol®, TBQ® | Less affected by organic material than other disinfectants. |
| Chlorhexidine | Nolvasan® , Hibiclens® | Remove organic matter prior to disinfection (organic matter reduces activity). |
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia for details of anesthesia procedures and monitoring requirements
- Administer appropriate analgesia at the time of anesthetic induction
- Refer to the IACUC Guidelines on Analgesia for further details
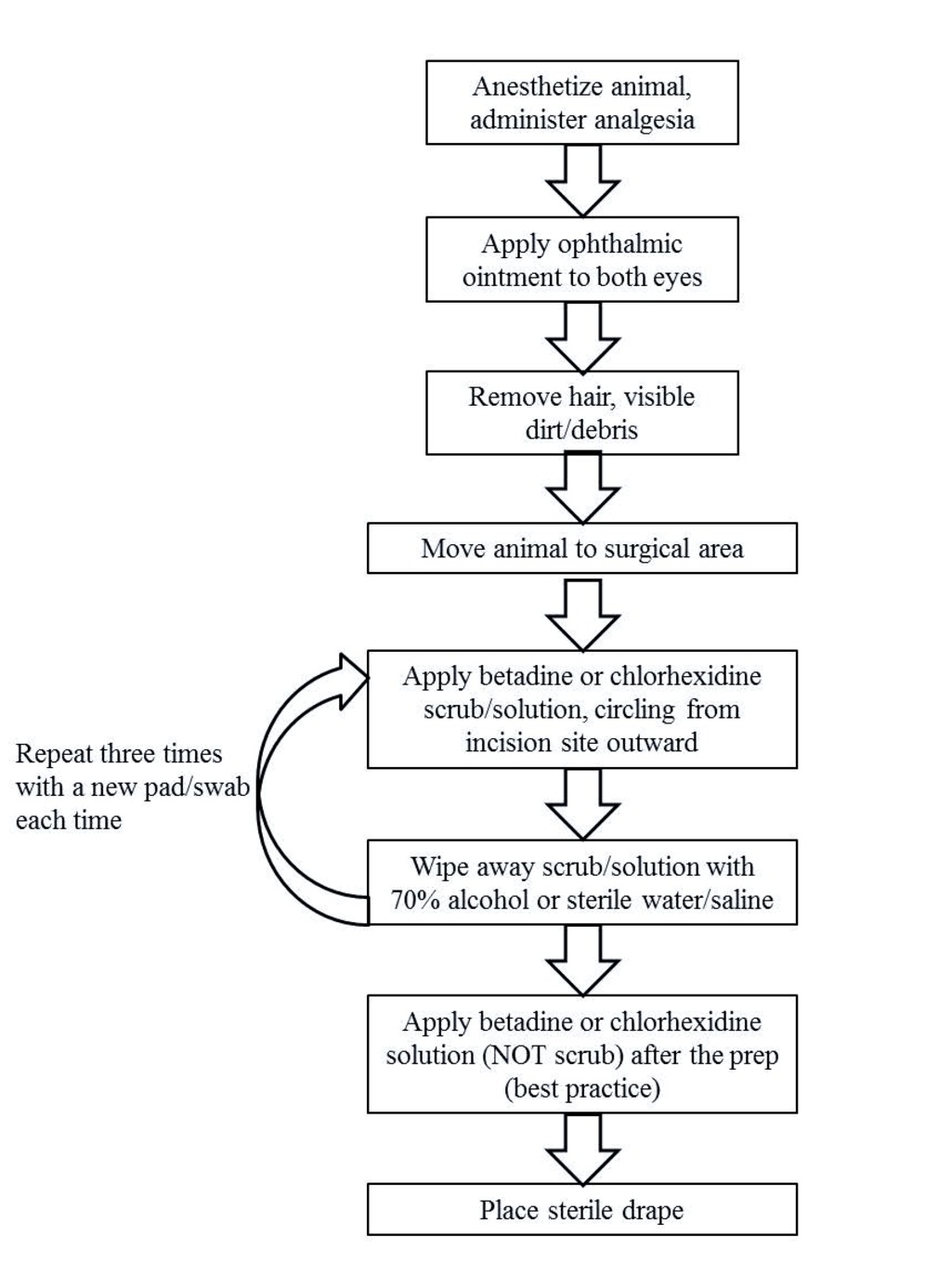
- Apply ophthalmic ointment to both eyes to prevent corneal desiccation
- Clip hair from the entire surgical field (everything that will not be covered by drapes)
- Remove loose hair and visible dirt/debris from the surgical site
- Move the animal from the prep room to the surgical table and secure the animal to the table
- Perform a surgical scrub of the incision site
- Note: alcohol alone is not sufficient for surgical preparation
- Utilize povidone-iodine ("betadine") or chlorhexidine for the surgical site preparation
- Available in two forms:
- "Solutions" which contain the antiseptic agent alone
- "Surgical scrubs" which contains a cleansing agent combined with the antiseptic agent
- Properly diluted antiseptic solutions may be left on the skin during surgery but surgical scrubs can be irritating and must be rinsed away after use
- Apply betadine or chlorhexidine surgical scrub or solution with clean gauze in a circular fashion starting at the surgical incision site and rotating outward
- Alternate surgical scrub or solution with 70% alcohol or sterile saline (best practice but not required unless using surgical scrub)
- Repeat a minimum of three times discarding cotton pad or swab after each use
- Apply betadine or chlorhexidine solution (NOT scrub) after the surgical prep scrub (best practice)
- Utilize povidone-iodine ("betadine") or chlorhexidine for the surgical site preparation
- Avoid excessive wetting of non-surgical areas of the animal with alcohol or disinfectant as this can exacerbate hypothermia
Optimal Surgical Scrub Prep Procedure:
Surgeon Preparation
- Surgeon and surgical assistant:
- Wear clean scrubs, shoe covers, surgical mask, and bonnet
- Perform surgical scrub of hands
- Put on sterile gown and gloves while maintaining sterility
- Observers/anesthetist:
- Wear clean scrubs, shoe covers, surgical mask, bonnet and disposable gloves
Aseptic Surgical Technique
- Place sterile drape on animal to prevent contamination of the incision, instruments and supplies
- No fur should be visible in the surgical field
- Unwrap the sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging (except with sterile gloves) as this will compromise the sterility of the instruments
- Maintain sterility of gloves and instruments throughout the surgery
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Care During Surgery
- Provide fluids (e.g., IV, IP, SQ) to maintain adequate hydration as described in the approved Animal Protocol
- Fluids should be provided during:
- major surgeries lasting longer than 30 minutes
- prolonged anesthesia for minor surgeries (and nonsurgical events, per Anesthesia Guidelines)
- Heat support (warming blanket, heated surgical stage/table, warmed IV fluids, etc.) is recommended for procedures/anesthesia lasting longer than 30 minutes
Postsurgical Care and Monitoring
- Assess the animals at least daily (including weekends and holidays) for at least five days post-operatively (days after surgery)
-
- Example 1, Surgical procedure occurs on Monday (day 0), the animals must be monitored at least daily Tuesday (day 1 post-op) through Saturday (day 5 post-op)
- Example 2, Surgical procedure occurs on Wednesday (day 0), the animals must be monitored at least daily Thursday (day 1 post-op) through Monday (day 5 post-op)
- Monitor animals for signs of pain Pain Recognition by Species:
- Monitor incision site for the following:
- Incisional integrity (i.e. sutures intact and wound is closed)
- Incision site infection: Heat, excessive swelling or purulent discharge
- Excessively tight sutures
- Remove sutures 7-14 days post-operatively
- Consult with OAR Veterinary staff if problems arise
Record Keeping
- Record the following information in addition to the anesthesia record requirements
- Note: the anesthesia record may be combined with the surgical record; a template can be found here
- PI Name, Animal Protocol Number
- Procedure
- Date, time, dose and route of each analgesia administration
- Date when wound closures (sutures, staples, clips) are removed/are no longer present
- Post-operative monitoring observations, including the date and time of each observation and a brief description of the animal’s health status and incision site appearance
- Reflect the animal’s health status by commenting on animal’s attitude, activity, hydration, appetite. For example:
- Attitude:
- Bright, alert and responsive (BAR)
- Quiet, alert and responsive (QAR)
- Depressed
- Activity
- Normal activity
- Decreased activity
- Appetite
- Normal appetite (normal feces, urine)
- Decreased appetite (decreased fecal and urine output?)
- Hydration:
- Normal hydration
- Dehydrated (increased skin tent)
- Attitude:
- Keep surgery, anesthesia and post-operative records readily accessible for review
Last Reviewed by the IACUC 10/11/2023