Breadcrumb
- Home
- Office of the IACUC
- Policies, Guidelines and Informational Sheets
- Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
Main navigation
- IACUC Contacts
- IACUC News
- IACUC AnShare ListServ
- Animal Protocol
-
Policies, Guidelines and Informational Sheets
- Anesthesia (Guideline)
- Analgesia - Buprenorphine ER (Informational Sheet)
- Analgesia (Guideline)
- Biologic Testing - Guidance and Procedures for Rodent Biologic Testing (Informational Sheet)
- Blood Collection (Guideline)
- Breeding - Rodent Breeding Colony Management (Policy)
- Enrichment - Canine Enrichment and Exercise Program
- Enrichment - Environmental Enrichment Program
- Euthanasia (Guideline)
- Euthanasia - Confirmation of Death (Policy)
- Euthanasia by OAR Personnel (Policy)
- Genotyping - Mouse Toe Clipping (Policy)
- Genotyping - Rodent Tail Snipping for Genotyping (Policy)
- Hazardous Agent Containment (biohazards, chemical hazards, & radioactive materials)
- Humane Intervention Points (Guideline)
- Media - Social Media (Policy)
- Media - Media Security (Policy)
- New Weanling Procedure for Labs (Guideline)
- Outbreak - OAR Pathogen Outbreak Control Plan
- Pain Recognition in Laboratory Animals (Informational Sheet)
- Principal Investigator Eligibility for Animal Protocols (Policy)
- Satellite Housing Expectactions (Policy)
- Social Housing of Species (Policy)
- Sterilization - Accepted Methods & Monitoring (IACUC Guideline)
- Substance Administration - Recommended Volumes (Informational Sheet)
- Substance Administration - Use of Drugs and Chemicals in Laboratory Animals (Guideline)
- Surgery - Non-Survival Surgery (Guideline)
- Surgery - Rodent (Mouse & Rat) Survival Surgery (Guideline)
- Surgery - Rodent Blood Loss (Informational Sheet)
- Surgery - USDA Covered Species Survival Surgery (Guideline)
- Surgery - Xenopus Oocyte Harvest (Guideline)
- Training Requirements for Personnel on an Animal Protocol (Policy)
- Transportation of Animals (Policy)
- Xenopus (Policy)
- Zebrafish (Policy)
- Personnel Training
- Educational Materials
- NIH Grant Information
- Occupational Hazards Associated with the Care and Use of Laboratory Animals
- DEA Information
- New Faculty/Recruitment
Guidelines: The IACUC has provided a set of guidance documents (Policies, Guidelines, and Informational Sheets) for use when planning animal procedures at the University of Iowa. An exception to a Guideline must be described and justified in the Animal Protocol and approved during the normal review process.
Purpose: The purpose of these guidelines is to provide direction for personnel conducting survival surgery on research rodents (mice of the genus Mus, rat of the genus Rattus) at the University of Iowa. These guidelines do not apply to USDA-covered species at the University of Iowa. These guidelines are intended for use by properly trained personnel listed on an IACUC-approved Animal Protocol who will be performing approved surgical procedures on rodent species (Mus, Rattus), or assisting with those procedures. Deviation from these guidelines must be described and justified in an IACUC-approved Animal Protocol.
Definitions:
Best Practice:
- Technique or method that consistently shows superior results
- Items in Guidelines labeled “best practice” are strongly recommended, but not required
Instrument and Equipment Sterilization
- All materials, implants, and substances used in surgery and/or placed inside the animals must be either sterile single-use (disposable) or sterilized prior to each use
- Start each day using only sterile instruments, surgical supplies and wound closure materials
- If a sterile instrument or sterile glove comes in contact with a non-sterile item, it is NO LONGER STERILE
- Re-sterilize or replace contaminated items before continuing aseptic procedures
- Re-sterilize or replace items before continuing aseptic procedures between animals
- Refer to sterilization methods for further information on material sterilization methods
- Sterile suture material and/or mechanical wound closures must be appropriate to the procedure and purpose
- Please consult an OAR veterinarian for guidance if you are unsure which closure material/method is appropriate for your purpose
- Synthetic non-absorbable monofilament suture (e.g. Prolene) is typically recommended for skin closures
- Silk sutures and other braided materials are undesirable for skin closures due to risk of wicking surface bacteria into the tissues. Use of silk sutures for skin closures must be described and scientifically justified in the Animal Protocol.
Surgery Location
- Allocate a clean uncluttered work area away from laboratory traffic, ventilation ducts and open windows
- Dedicate the area solely to surgical procedure(s) when in use
- Utilize a separate room used primarily for aseptic procedures, if possible
Area Preparation
- Clean work surface with a disinfectant
- Apply a clean drape over the working surface where the surgery will be performed
- Establish a sterile field near the animal for placement of sterile instruments (see Figure 1)
Figure 1: Surgical Area Preparation
A) Common supplies for survival rodent surgery:
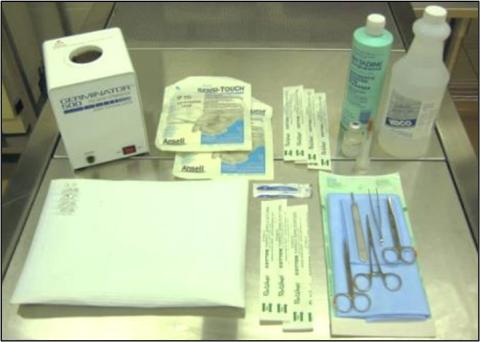
A) Common supplies for survival rodent surgery: disinfectant wipes or spray, hot bead sterilizer, two pairs of sterile gloves, a clean drape (the surgical area), several sterile cotton-tipped applicators for sterile preparation of the surgical site and hemostasis, povidine-iodine or chlorhexidine scrub and 70% ethanol, sterile saline, insulin syringes, a scalpel blade, and a sterile surgical pack and instrument stand.
B) Sterile-tip instrument set-up
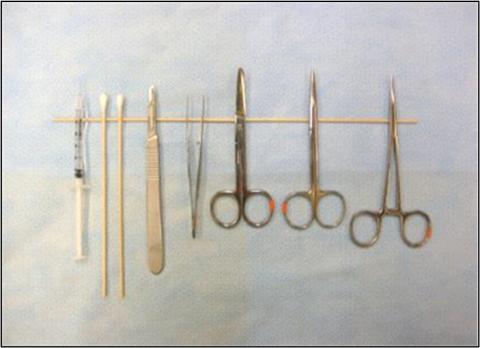
B) Sterile-tip Instrument Setup - Instruments are positioned with the hinge resting on the instrument stand (sterile wooden dowel, or other sterile dividing material), which divides the underlying drape into upper STERILE and lower NON-STERILE areas. This setup ensures maintenance of instrument tip sterility in spite of contaminated handles when using sterile “instrument tip” technique. The use of an instrument stand maintains the instrument tips at an elevated position (insert), reducing surface contact and promoting sterility.
C) Surgical Set-up
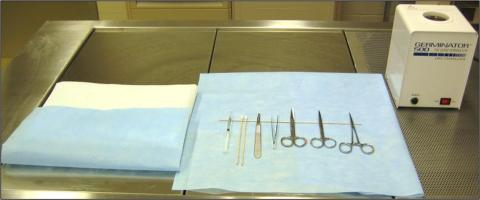
C) Surgical Setup - The animal is positioned on the surgical field (with heat support) and draped. Instruments are placed on the instrument drape in an organized fashion, utilizing the instrument stand.
Examples of hard surface disinfectants
| AGENT | EXAMPLES | COMMENTS |
|---|---|---|
| Quaternary Ammonium | Sani Cloth®, Roccal®, Quatricide®, Tec-Surf II® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorine | Sodium hypochlorite (Clorox® 10% solution) Chlorine dioxide (Clidox®, MB-10®) | Remove organic matter prior to disinfection (organic matter reduces activity). Note: Solutions need to be made up fresh daily to maintain activity. |
| Glutaraldehydes | Glutaraldehydes (Cetylcide®, Cide Wipes®) | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Phenolics | Lysol®, TBQ® | Remove organic matter prior to disinfection (organic matter reduces activity). |
| Chlorhexidine | Nolvasan® , Hibiclens® | Remove organic matter prior to disinfection (organic matter reduces activity). |
Animal Preparation
- Anesthetize the animal in accordance with the approved Animal Protocol
- Refer to the IACUC Guidelines on Anesthesia for details of anesthesia agents, procedures, and monitoring requirements
- Administer appropriate analgesia at the time of anesthetic induction
-
- Refer to the IACUC Guidelines on Analgesia for further details
- Apply ophthalmic ointment to both eyes to prevent corneal desiccation
- Remove hair from the surgical site using one of the following:
- Clipper blade
- Depilatory cream
- Fur plucking
- Remove loose hair and visible dirt/debris from the surgical site
- Perform a surgical site preparation of the incision site.
- Note: alcohol alone is not sufficient for surgical preparation
- Utilize povidone-iodine ("betadine") or chlorhexidine antiseptic products for the surgical site preparation
- Available in two forms:
- "Solutions" which contain the antiseptic agent alone
- "Surgical scrubs" which contain a cleansing agent combined with the antiseptic agent
- Properly diluted antiseptic solutions may be left on the skin during surgery but surgical scrubs can be irritating and must be rinsed away after use
- Available in two forms:
- Apply betadine or chlorhexidine surgical scrub or solution with clean gauze in a circular fashion starting at the surgical incision site and rotating outward
- Alternate surgical scrub or solution with 70% alcohol or sterile saline (best practice but not required unless using surgical scrub)
- Repeat a minimum of three times discarding cotton pad or swab after each use
- Apply betadine, chlorhexidine solution (NOT scrub), or end with a final application of alcohol or sterile saline after the surgical prep
- Avoid excessive wetting of non-surgical areas of the animal with alcohol or antiseptic as this can exacerbate hypothermia
- Cover rodent with sterile drape or Glad Press and Seal to avoid contamination of the incision, instruments and supplies
- It is recommended to use clear drapes to facilitate observation of the rodent
Optimal Surgical Prep Procedure:

Aseptic Surgical Technique
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Unwrap sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging or instruments as this will compromise the sterility of the instruments
- Perform a surgical scrub of the surgeon’s hands
- Put on sterile gloves without touching the exterior of the glove surface
- Use the sterile interior of the glove packaging as a sterile field for instruments
- Maintain sterility of gloves and instruments throughout the surgery
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling sterile suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Aseptic Tip Technique
- Put on a clean lab coat or scrub top, surgical mask and tie back or cover long hair
- Start with sterile instruments at the beginning of each set of distinct surgical procedures
- Unwrap sterile instruments making sure to only touch the outer surface of the wrap
- Do not touch the interior of the packaging or instruments as this will compromise the sterility of the instruments
- Wash hands or perform surgical scrub
- Put on sterile or procedural gloves
- The interior of sterile glove packaging may be used as a sterile field for instruments if sterile gloves are used
- Arrange the sterile instruments so that the tips are within a sterile field and the handles are outside the sterile field
- Do not contaminate the sterile tips of the instruments during this process
- Instrument tips must be maintained within this sterile field throughout the surgery
- Utilize only the sterile tips of the instruments inside the body cavity
- Tips of surgical instruments must be sterilized between animals or multiple surgeries. (see Figure 1)
- Maintain sterile suture material within the sterile field at all times
- Avoid pulling suture across non-sterile areas (e.g., across animal’s body, areas surrounding the sterile field)
Postsurgical Care and Monitoring
- Immediately post-operatively, evaluate the animal’s surgical blood loss and provide replacement fluids if appropriate. (See Informational Sheet for estimation guidance)
- Commercial (pharmaceutical grade) sterile saline for injection (NaCl) or lactated ringer’s solution may be given subcutaneously in a volume equal to the estimated blood loss.
- Recovery the animal from anesthesia according to the IACUC Guidelines on Anesthesia
- Assess the animals at least daily (including weekends and holidays) for at least five days post-operatively (days after the surgery) or as justified in the Animal Protocol
-
- Example 1, Surgical procedure occurs on Monday (day 0), the animals must be monitored at least daily Tuesday (day 1 post-op) through Saturday (day 5 post-op)
- Example 2, Surgical procedure occurs on Wednesday (day 0), the animals must be monitored at least daily Thursday (day 1 post-op) through Monday (day 5 post-op)
- Monitor animals for signs of pain Pain Recognition by Species and contact OAR Veterinary Staff if signs are observed
- Monitor incision site for the following:
- Incisional integrity (i.e. sutures intact and wound is closed)
- Incision site infection: Heat, excessive swelling or purulent discharge
- Excessively tight sutures
- Consult with Office of Animal Resources Veterinary staff if above signs or other problems arise
- Including potential re-closure of surgical site dehiscence/wound closure failure
- Remove wound closure 7-14 days post-operatively
Record Keeping
- Record the following information in addition to the anesthesia record requirements
- Note: the anesthesia record may be combined with the surgical record; a template can be found here
- Animal Protocol number, PI name, individual performing procedure
- Procedure
- Date, time, dose and route of each analgesia administration
- Date when wound closures (sutures, staples, clips) are removed/are no longer present
- Post-operative monitoring observations, including the date and time of each observation and a brief description of the animal’s health status and incision site appearance
- Reflect the animal’s health status by commenting on animal’s appearance, posture and activity.
- Keep surgery, anesthesia and post-operative records readily accessible for review
Last reviewed by the IACUC: 10/11/2023